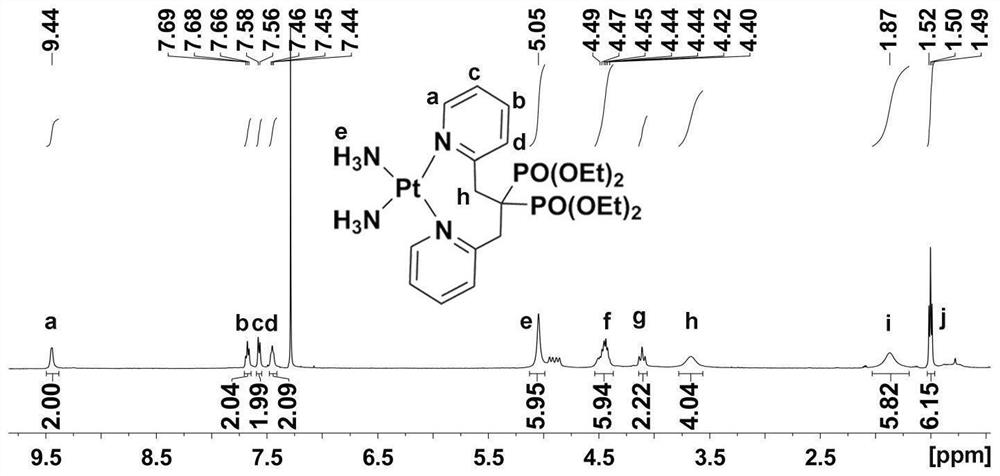
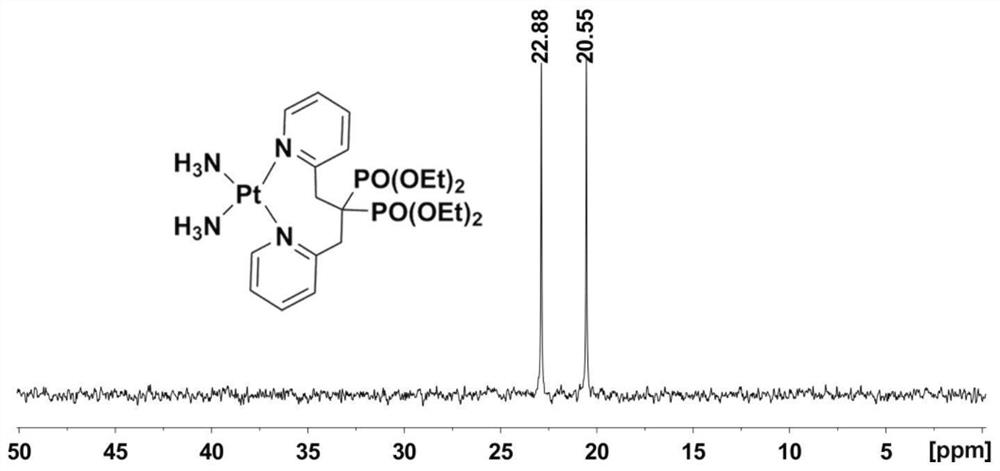
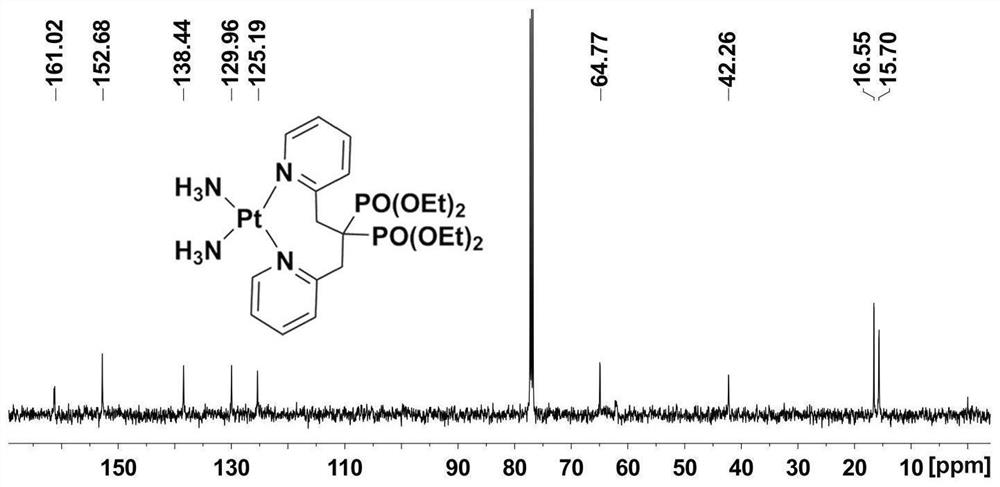
Platinum diphosphonate complex as well as synthesis method and application thereof
A technology of platinum complexes and synthesis methods, applied in the field of chemical biology, can solve the problems of reduced curative effect and achieve the effects of alleviating drug resistance, high synthesis yield and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The synthetic method of bisphosphonic acid platinum complex, comprises the steps:
[0041] S1: Synthesis of 2-chloromethylpyridine:
[0042] 2-Chloromethylpyridine hydrochloride (5.292g, 32mmol) was dissolved in distilled water (7mL), and sodium hydroxide (1.344g, 32mmol) was added to distilled water (14mL) to make an alkaline solution, and the above two solutions were After mixing, extraction was performed with chloroform, and the extracted chloroform layer was dried over anhydrous magnesium sulfate and the solvent was removed in vacuo. The filter residue was purified by distillation under reduced pressure to obtain light red 2-chloromethylpyridine.
[0043] S2: Synthesis of [1,3-bis(2-pyridyl)propane-2,2-diyl]bis(phosphonic acid)tetraethyl ester:
[0044] Under nitrogen protection conditions at 273K, 20 mL of anhydrous DMSO added with 2-[2-pyridylethylethyl] bis(phosphonic acid) tetraethyl ester (9.31 g, 24.56 mmol) was added to 60% NaH (1.08 g , 29.47mmol) in 20mL...
Embodiment 2
[0048] Explore the reactivity of the bisphosphonate platinum complex DBPP and GSH prepared in Example 1, the steps are as follows:
[0049] The bisphosphonate platinum complex DBPP (0.0056mmol) was dissolved in deuterium water and phosphate buffer solution (PBS7.87mM, pH 7.4, D 2 (0.5 mL) of the mixed solution, put the above liquid into the nuclear magnetic resonance tube. Add GSH (2.45mg, 0.0132mmol) to the same nuclear magnetic resonance tube, place the nuclear magnetic resonance tube in a 310K water bath for incubation, and use it at different time intervals 31 P NMR and 1 H NMR detects the solution in the tube, and the test results are shown in the attached Image 6 .
[0050] attached Image 6 a The data shows that the peak values of DBPP are located at 24.88 and 20.09ppm respectively, and after 168h of cultivation, no significant change in the data has been observed, which shows that DBPP has a certain inertia to glutathione; attached Image 6 Similar results can ...
Embodiment 3
[0052] Explore the lipophilicity of the bisphosphonic acid platinum complex DBPP prepared in Example 1, the steps are as follows:
[0053] The lipophilicity coefficient was determined by n-octanol shake flask method. Add (50, 100, 150, 200μM) DBPP solutions to 10mM, pH 7.4 phosphate buffer solution respectively, mix 2.0mL of the above solutions with an equal volume of 1-octanol, and place on a constant temperature (25.0±0.1℃) air bath orbital shaker Centrifuge at 200rpm for 4h. After centrifuging at 2500rpm for 15min, the sample was divided into two phases; max =267nm) to measure the DBPP concentration in the aqueous solution, calculate the logarithmic P value of the DBPP concentration and the DBPP concentration in the n-octanol phase to obtain the fat-water partition coefficient of DBPP, the measurement results refer to the appendix Figure 7 , see the attached table 1 for the calculation results.
[0054] attached Figure 7 The data showed a regular distribution of DBPP ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com