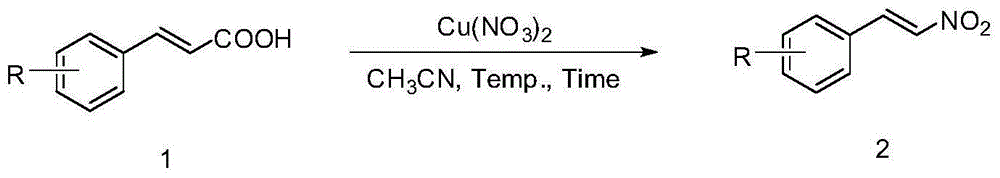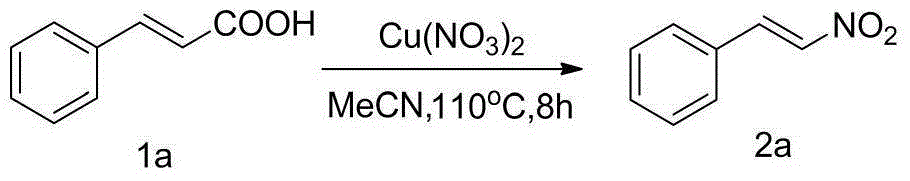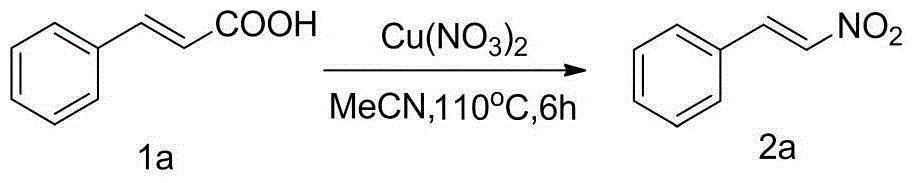Method for preparing beta-nitrostyrolene compound
A technology of nitrostyrene and compounds, which is applied in the field of preparing β-nitrostyrene compounds, can solve the problems of not having good economic benefits, low yield, long reaction time, etc., and achieves wide application range of substrates, The effect of simple post-treatment process and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Implementation Example 1: Add 0.148g (1mmol) cinnamic acid 1a, 0.241g (1mmol) copper nitrate, and 6mL acetonitrile into a 35ml thick-walled pressure-resistant tube in sequence, and in an oil bath at 110°C, magnetically stir the reaction for 8h and monitor by TLC reaction process. After the reaction was completed, column chromatography separated [petroleum ether (60~90° C.) / ethyl acetate=4 / 1] to obtain β-nitrobenzene 2a, 139 mg of yellow solid, and the yield was 93%, Mp: 55- 58°C. 1 HNMR (400MHz, CDCl 3 )δ8.05(d,J=10.8Hz,2H),7.79–7.23(m,4H),1.66(s,1H); 13 CNMR (100MHz, CDCl 3 ) δ139.1, 137.2, 132.2, 130.1, 129.4, 129.2. The reaction principle of implementation example 1 is as follows:
[0021]
Embodiment 2
[0022] Implementation example 2: Add 0.148g (1mmol) cinnamic acid 1a, 0.241g (1mmol) copper nitrate, and 6mL acetonitrile into a 35ml thick-walled pressure-resistant tube in sequence, and in an oil bath at 110°C, magnetically stir the reaction for 6h and monitor by TLC reaction process. After the reaction, separated by column chromatography [petroleum ether (60-90° C.) / ethyl acetate=4 / 1] to obtain β-nitrostyrene 2a, 131 mg of a yellow solid, with a yield of 88%. The reaction principle of implementation example 2 is as follows:
[0023]
Embodiment 3
[0024] Implementation example 3: Add 0.148g (1mmol) cinnamic acid 1a, 0.241g (1mmol) copper nitrate, and 6mL acetonitrile into a 35ml thick-walled pressure-resistant tube in sequence, and in an oil bath at 110°C, magnetically stir the reaction for 10h and monitor by TLC reaction process. After the reaction, separated by column chromatography [petroleum ether (60-90° C.) / ethyl acetate=4 / 1] to obtain β-nitrostyrene 2a, 127 mg of yellow solid, with a yield of 85%. The reaction principle of implementation example 3 is as follows:
[0025]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com