Porous organic polymer linked by thiourea structure as well as preparation method and application of porous organic polymer
A technology of structural connection and polymer, applied in the preparation of organic compounds, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve the problem of limited modifiability and designability, limited application range, difficult Satisfies the problems of diversified catalytic systems, and achieves the effects of easy recycling, good solvent tolerance, and reduced catalyst consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The synthesis of embodiment 1 porous organic polymer POP-S
[0048] Dissolve 145mg (0.5mmol) of compound II tris(4-aminophenyl)amine and 144mg (0.75mmol) of compound III p-phenylene diisothiocyanate in 5mL of N,N-dimethylformaldehyde under argon atmosphere Amide, add compound II solution dropwise to compound III solution, ultrasonically disperse for 3 minutes, freeze and deoxygenate 3 times; heat to 60°C, react for 72 hours, cool to room temperature, add 50mL acetone, stir for 1 hour, filter, Washing with dichloromethane, acetone, methanol, and water in sequence was repeated 3 times to remove unreacted monomers. The resulting solid product was vacuum-dried at 80° C. for 24 hours to obtain 216 mg of brown solid POP-S (yield 74.7%).
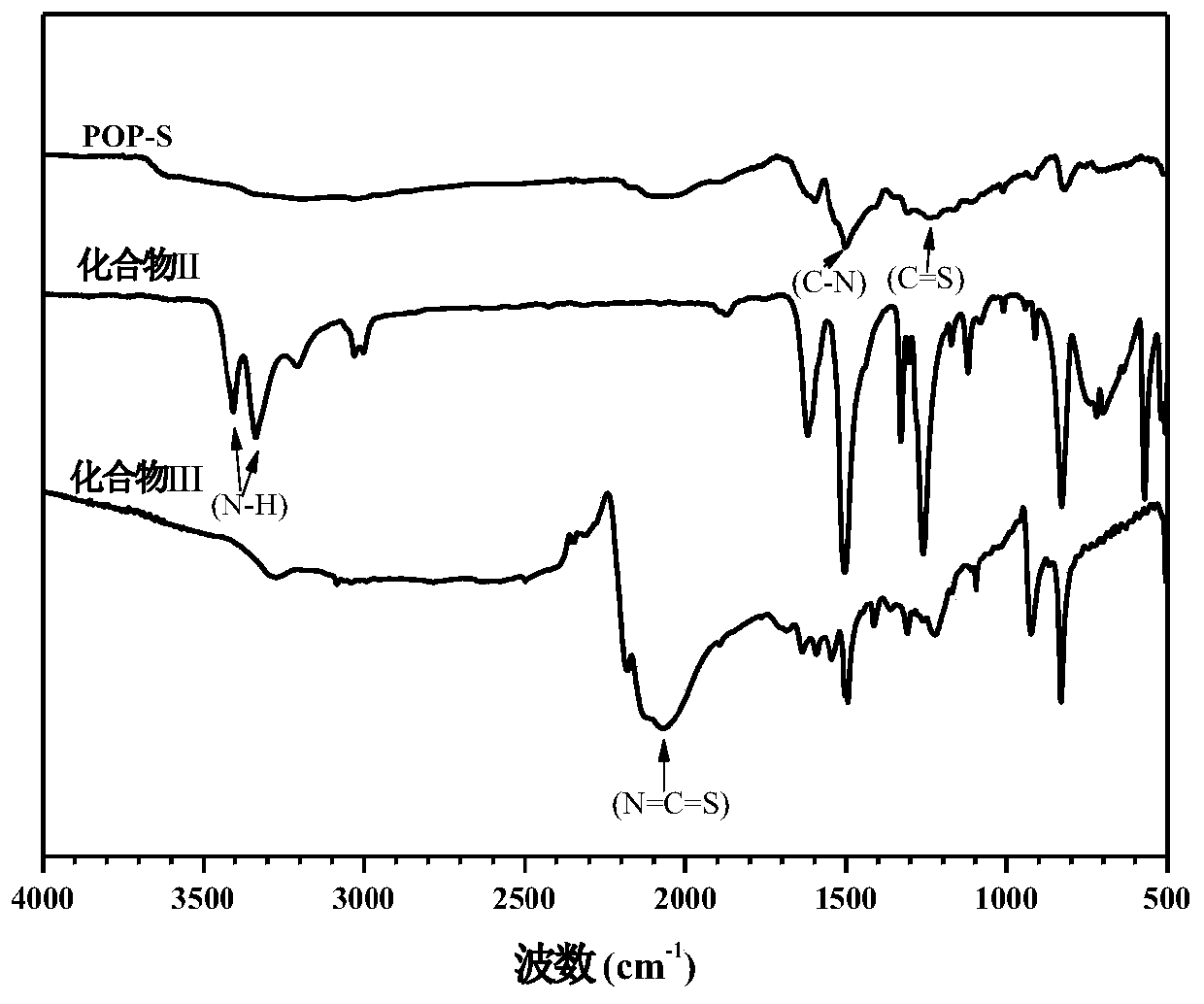
[0049] figure 1 Infrared spectra of the porous organic polymer POP-S prepared in Example 1 and its reactants, namely compound II and compound III. Comparing the infrared spectra of these three compounds, it can be seen that compound II is ...
Embodiment 2
[0056] The synthesis of embodiment 2 porous organic polymer POP-S
[0057] Dissolve 145mg (0.5mmol) of compound II tris(4-aminophenyl)amine and 144mg (0.75mmol) of compound III p-phenylene diisothiocyanate in 5mL of N,N-dimethylformamide under nitrogen atmosphere , add compound II solution dropwise to compound III solution, ultrasonically disperse for 3 minutes, freeze and deoxygenate 3 times; heat to 120°C, react for 48 hours, cool to room temperature, add 50mL acetone, stir for 1 hour, filter, and then Washing with dichloromethane, acetone, methanol, and water was repeated 3 times to remove unreacted monomers. The resulting solid product was vacuum-dried at 80° C. for 24 hours to obtain 219 mg of brown solid POP-S (yield 75.8%).
[0058]
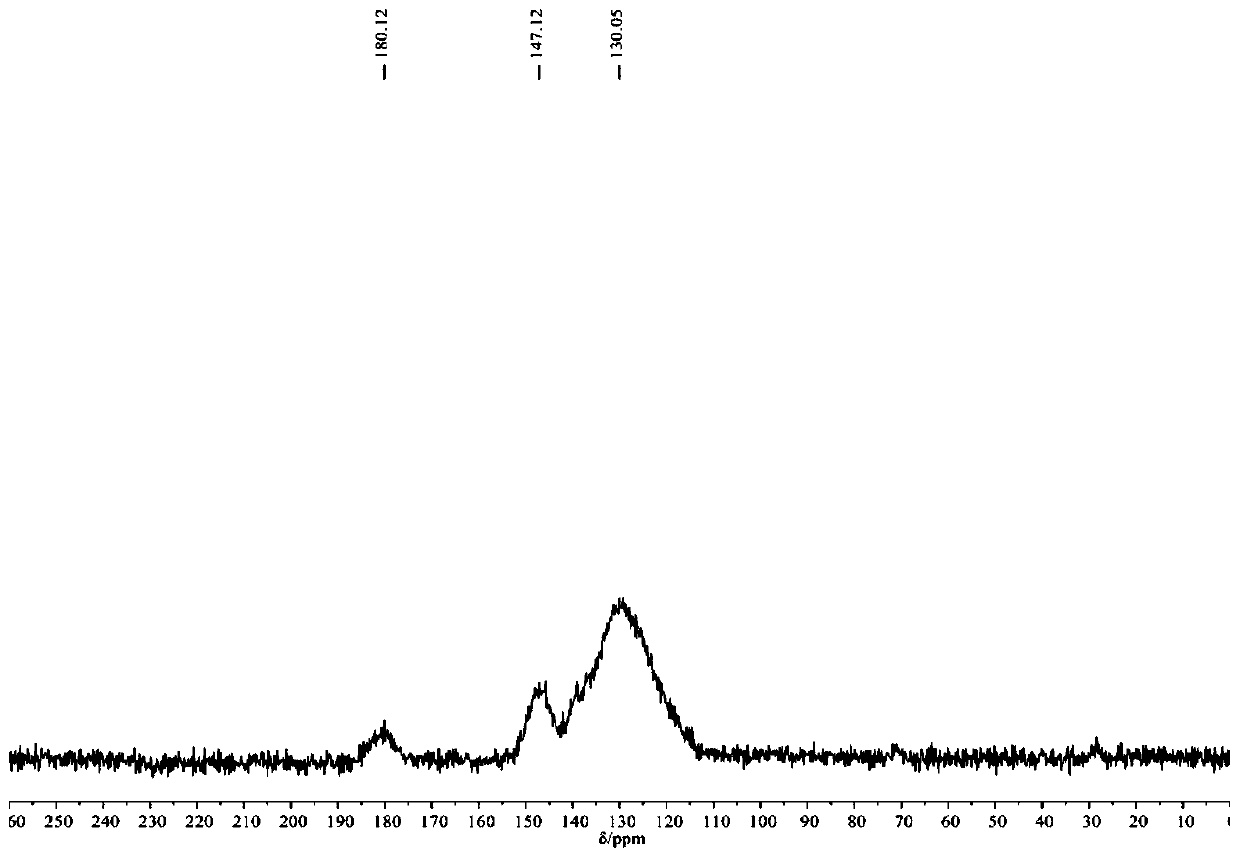
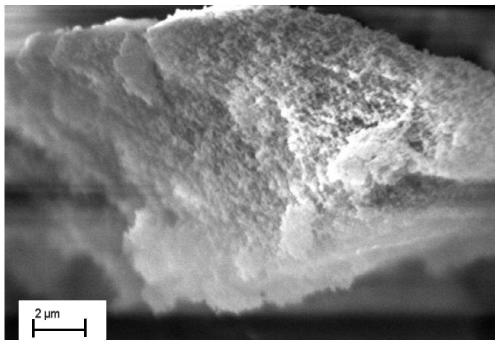
[0059] The infrared spectrogram and the solid-state nuclear magnetic spectrogram of the porous organic polymer POP-S prepared by the present embodiment are respectively compared with figure 1 , figure 2 Consistent, its scanning elect...
Embodiment 3
[0060] The synthesis of embodiment 3 porous organic polymer POP-S
[0061] Dissolve 145mg (0.5mmol) of compound II tris(4-aminophenyl)amine and 144mg (0.75mmol) of compound III p-phenylene diisothiocyanate in 5mL of ethanol under an argon atmosphere, and add the compound II solution dropwise into the compound III solution, ultrasonically disperse for 3 minutes, freeze and deoxygenate 3 times; heat to 60°C, react for 72 hours, cool to room temperature, filter, wash with dichloromethane, acetone, methanol, and water successively, repeat 3 times, Unreacted monomers were removed, and the obtained solid product was vacuum-dried at 80° C. for 24 hours to obtain 232 mg of brown granular solid POP-S (yield 80.3%).
[0062]
[0063] The infrared spectrogram and the solid-state nuclear magnetic spectrogram of the porous organic polymer POP-S prepared by the present embodiment are respectively compared with figure 1 , figure 2 Consistent, no longer repeat; its scanning electron mic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com