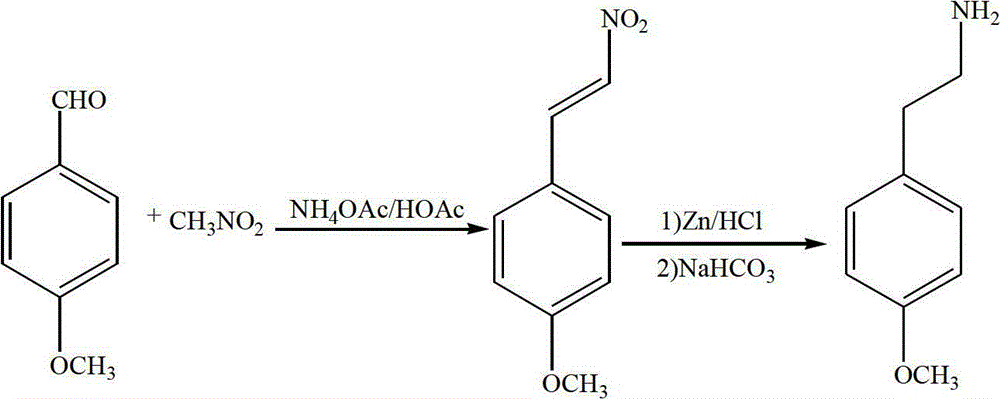
Preparation method of 4-methoxy-beta-phenylethylamine
A technology of methoxybenzaldehyde and methoxy, which is applied in the field of preparation of 4-methoxy-β-phenethylamine, can solve the problems of cumbersome reaction steps, low reaction yield, difficult industrialization and the like, and achieves reactivity High, simple operation steps, short response time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 1) Preparation of 4-methoxy-β-nitrostyrene
[0018] In the reactor, add 20.4g (0.15mol) 4-methoxybenzaldehyde, 20.1g (0.33mol) nitromethane, 14.0g (0.18mol) ammonium acetate and 75ml (1.3mol) glacial acetic acid, stir and heat to reflux, After reacting for 4~4.5h, the reaction solution was cooled to 12~15°C, then poured into ice water at -2~2°C, yellow needle-like crystals were precipitated, filtered with suction, the filter cake was washed with water, and dried to obtain 4-methoxy- β-nitrostyrene is a yellow solid, and then recrystallized with tetrahydrofuran alcohol to obtain 23.5 g of yellow crystals, yield: 87.3%, m.p.: 85~87 °C;
[0019] 2) Preparation of 4-methoxy-β-phenethylammonium
[0020] Add 64.9g (1.0mol) of zinc powder into the reaction, then add 10~30Wt.% hydrochloric acid aqueous solution, the amount added is less than the zinc powder, stir evenly, let stand for 0.5~1h, filter to obtain activated zinc powder;
[0021] In the reactor, add activated zinc ...
Embodiment 2
[0023] 1) Preparation of 4-methoxy-β-nitrostyrene
[0024] In the reactor, add 20.4g (0.15mol) 4-methoxybenzaldehyde, 24.7g (0.45mol) nitromethane, 16.4g (0.21mol) ammonium acetate and 82ml (1.4mol) glacial acetic acid, stir and heat to reflux, After reacting for 4~4.5h, the reaction solution was cooled to 10~15°C, then poured into ice water at -2~2°C, yellow needle-like crystals were precipitated, filtered with suction, the filter cake was washed with water, and dried to obtain 4-methoxy- β-Nitrostyrene is a yellow solid, and then recrystallized from tetrahydrofuran alcohol to obtain 23.8g of yellow crystals, yield: 88.5%, m.p.: 85~87°C;
[0025] 2) Preparation of 4-methoxy-β-phenethylammonium
[0026] Add 54.5g (0.84mol) of zinc powder into the reaction, then add 10~30Wt.% hydrochloric acid aqueous solution, the amount added is less than the zinc powder, stir evenly, let stand for 1h, and filter to obtain activated zinc powder;
[0027] In the reactor, add activated zinc p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com