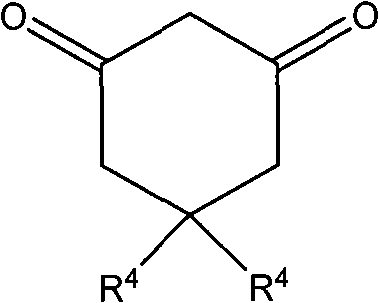
Synthesis method of 2-nitro-3-aryl-2,3,5,7-tetrahydrobenzofuran-4-one derivative
A technology of benzofuran and nitrofurane, which is applied in directions such as organic chemistry, can solve the problems of few reaction substrates, high product price, inconsistency, etc., and achieves simplified reaction operation and post-processing process, easy availability of raw materials, The effect of reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Dissolve 1 mmol of β-nitrostyrene, 2 mmol of manganese acetate, and 1 mmol of 1,3-cyclic diketone in 10 ml of water, 95% ethanol, 80% ethanol, 50% ethanol, absolute ethanol, and acetic acid , acetonitrile, and the reaction solution is placed in a water bath and heated to 60 ° C, TLC tracking reaction shows that the best reaction effect (86% yield) in 95% ethanol, the results are as follows:
[0049] solvent
Embodiment 2
[0050] Example 2: Dissolve 1 mmol of β-nitrostyrene, 2 mmol of manganese acetate, and 1 mmol of 1,3-cyclic diketone in 10 ml of 95% ethanol at 20°C, 30°C, 40°C, 50°C, and 60°C, respectively. Reaction, TLC follow-up reaction shows that reaction effect is the best (productive rate 86%) at 50 ℃, the result is as follows:
[0051] temperature(℃)
Embodiment 3
[0052] Example 3: 1 mmol of β-nitrostyrene, 2 mmol of manganese acetate, and 1 mmol, 2 mmol, and 3 mmol of 1,3-cyclic diketone were respectively dissolved in 95% ethanol and reacted at 50° C. TLC tracking reaction showed 1, When the amount of 3-cyclic diketone is 2mmol, the reaction yield is the highest (productive rate 86%), and the results are as follows:
[0053] Manganese acetate (mmol)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com