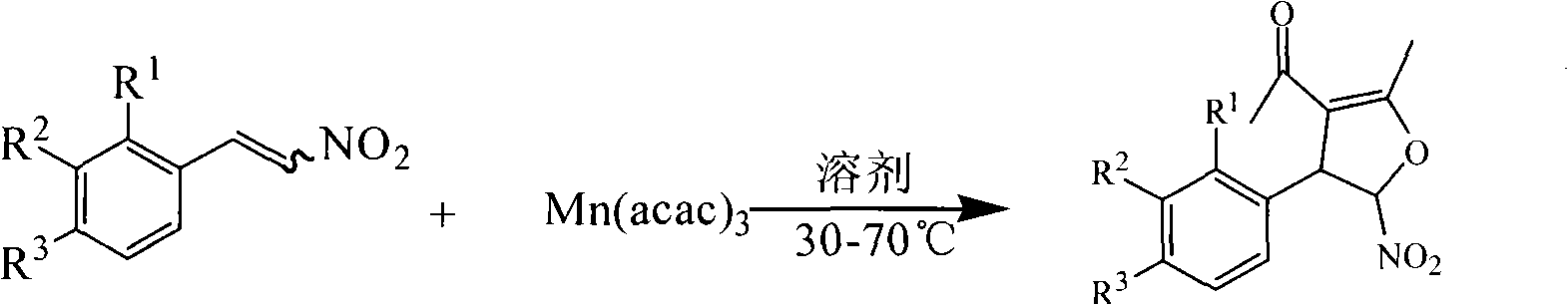
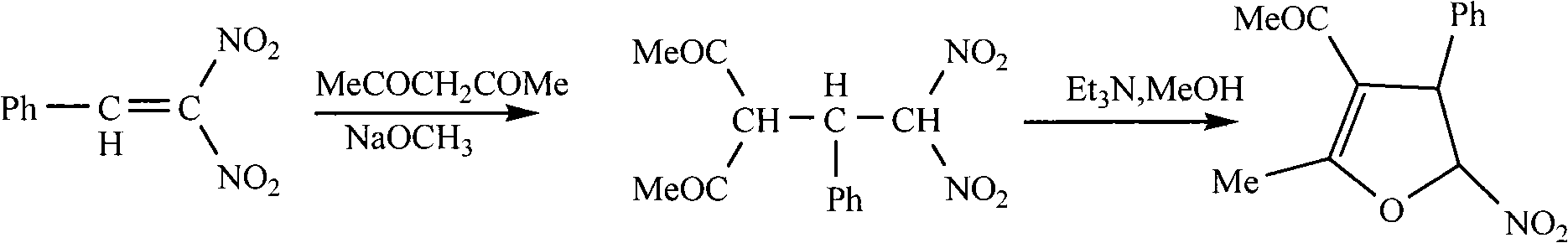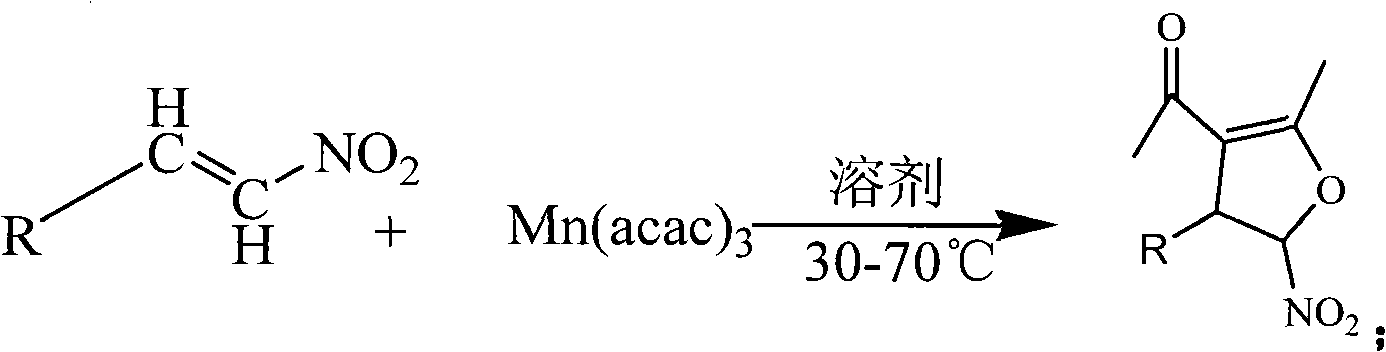Method for synthesis of 5-nitryl-4, 5-dihydrofuran derivant
A technology of dihydrofuran and nitrofuran ethylene, which is applied in the direction of organic chemistry, can solve the problems of few types of products, harsh reaction conditions, complex reactions, etc., and achieve the effects of shortened reaction time, simple synthesis, and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Dissolve 1 mmol of β-nitrostyrene and 1 mmol of manganese (III) acetylacetonate in 10 ml of anhydrous methanol, absolute ethanol, acetonitrile, chloroform, dichloromethane, 1,2-dichloroethane, toluene respectively , and the reaction solution was placed on a water bath and heated to 80°C. TLC tracking of the reaction showed that the reaction effect was best in absolute ethanol.
Embodiment 2
[0050] Example 2: Dissolve 1 mmol of β-nitrostyrene and 1 mmol of manganese (III) acetylacetonate in 10 ml of absolute ethanol, respectively at room temperature, 30°C, 40°C, 50°C, 55°C, 60°C, 70°C, Reaction at 80°C and 90°C, TLC tracking reaction showed that the best reaction effect was at 55°C.
Embodiment 3
[0051] Example three: 1 mmol of β-nitrostyrene and 1 mmol, 2 mmol, 3 mmol, and 4 mmol of manganese (III) acetylacetonate were dissolved in absolute ethanol and reacted at 55° C. TLC tracking reaction showed that the manganese (III) acetylacetonate ) is the highest reaction yield when the amount of 3mmol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com