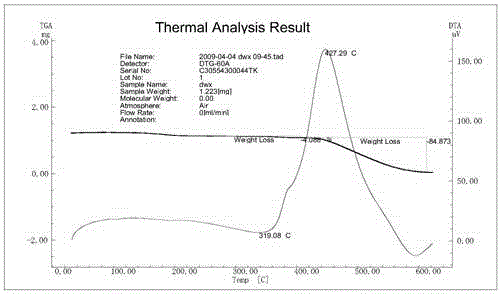
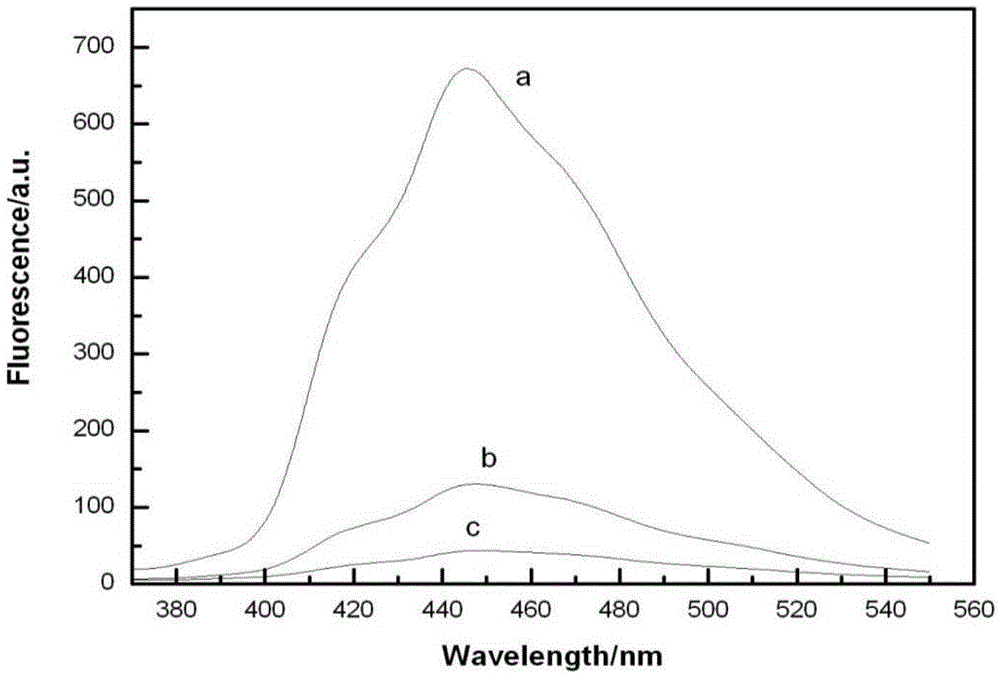
Fullerene pyrrolidine derivative and preparation method thereof
A technology of fullerene pyrrolidine and derivatives, which is applied in the preparation of organic compounds, preparation of amino hydroxyl compounds, chemical instruments and methods, etc., and can solve problems such as limited application, poor solubility, and unsatisfactory targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The method for preparing 3-(4-nitrophenyl)acrolein described in this embodiment comprises the following steps:
[0033] Get 5.0g (66mmol) of p-nitrobenzaldehyde and 10.0mL of acetaldehyde into the three-necked flask, add 10mL of 20% potassium hydroxide-methanol solution in the dropping funnel, stir magnetically, and cool the reaction with an ice-salt bath bottle, when the temperature drops to 0-5°C, slowly add 5mL potassium hydroxide-methanol solution dropwise under magnetic stirring, react for 40min, then add 5mL potassium hydroxide-methanol solution dropwise, continue to react at about 10°C for 40min, cool The reaction mixture solidifies below 5°C, add 16 mL of acetic anhydride, heat on a water bath for 30 min, pour into 120 mL of hot water, add 16 mL of concentrated hydrochloric acid, heat in a water bath for 20 min, and let stand for 24 h. Crystals were precipitated, filtered by suction, washed, and recrystallized in 30% aqueous acetic acid solution by mass fraction...
Embodiment 2
[0040] The method for preparing 3-(4-nitrophenyl)acrolein described in this embodiment comprises the following steps:
[0041] Get 5.0g (66mmol) p-nitrobenzaldehyde and 10.0mL acetaldehyde into the three-necked flask, add 10mL mass fraction of 10% potassium hydroxide-methanol solution in the dropping funnel, stir magnetically, and cool the reaction with an ice-salt bath bottle, when the temperature drops to 0-5°C, slowly add 5mL potassium hydroxide-methanol solution dropwise under magnetic stirring, react for 40min, then add 5mL potassium hydroxide-methanol solution dropwise, continue to react at about 10°C for 40min, cool The reaction mixture solidifies below 5°C, add 16 mL of acetic anhydride, heat on a water bath for 30 min, pour into 120 mL of hot water, add 16 mL of concentrated hydrochloric acid, heat in a water bath for 20 min, and let stand for 24 h. Crystals were precipitated, suction filtered, washed, and recrystallized in 30% aqueous acetic acid solution to obtain 1...
Embodiment 3
[0043] The method for preparing 3-(4-nitrophenyl)acrolein described in this embodiment comprises the following steps:
[0044] Get 5.0g (66mmol) of p-nitrobenzaldehyde and 10.0mL of acetaldehyde into the three-necked flask, add 10mL of 30% potassium hydroxide-methanol solution in the dropping funnel, stir magnetically, and cool the reaction with an ice-salt bath bottle, when the temperature drops to 0-5°C, slowly add 5mL potassium hydroxide-methanol solution dropwise under magnetic stirring, react for 40min, then add 5mL potassium hydroxide-methanol solution dropwise, continue to react at about 10°C for 40min, cool The reaction mixture solidifies below 5°C, add 16 mL of acetic anhydride, heat on a water bath for 30 min, pour into 120 mL of hot water, add 16 mL of concentrated hydrochloric acid, heat in a water bath for 20 min, and let stand for 24 h. Crystals were precipitated, filtered with suction, washed, and recrystallized in 30% aqueous acetic acid solution to obtain 1.6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com