Synthetic method for 4-amino-3-phenylbutyric acid hydrochloride
A technology of phenylbutyrate hydrochloride and synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., and can solve problems such as high price of starting materials, heavy pollution in the production process, and cumbersome operation steps , to achieve the effect of low cost, high yield and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
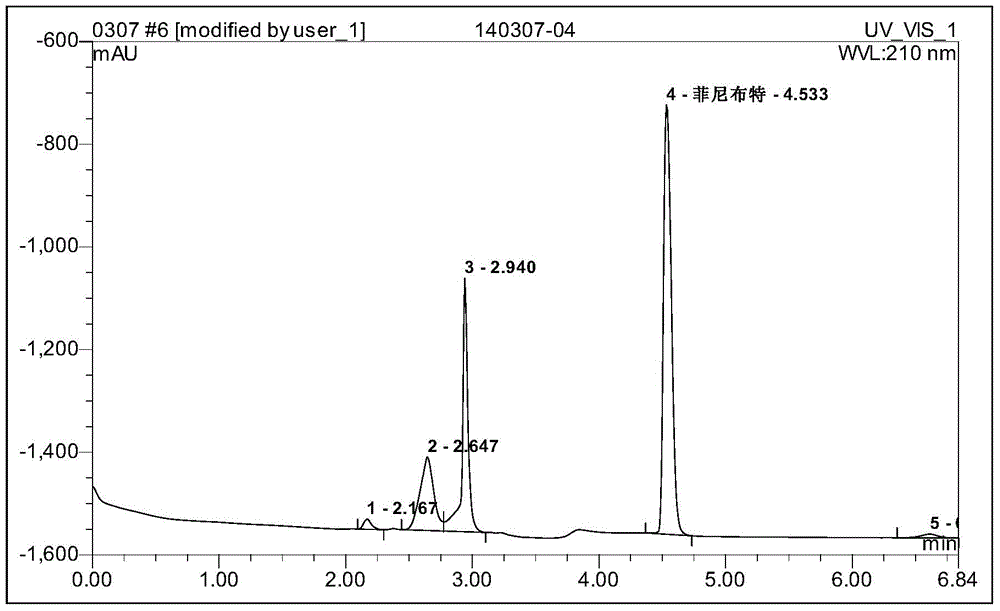
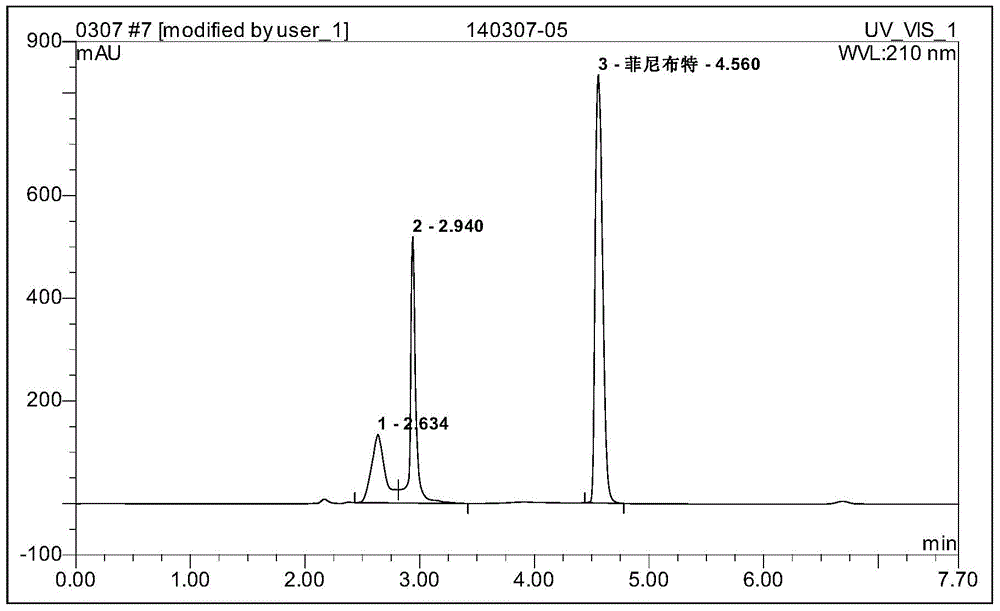
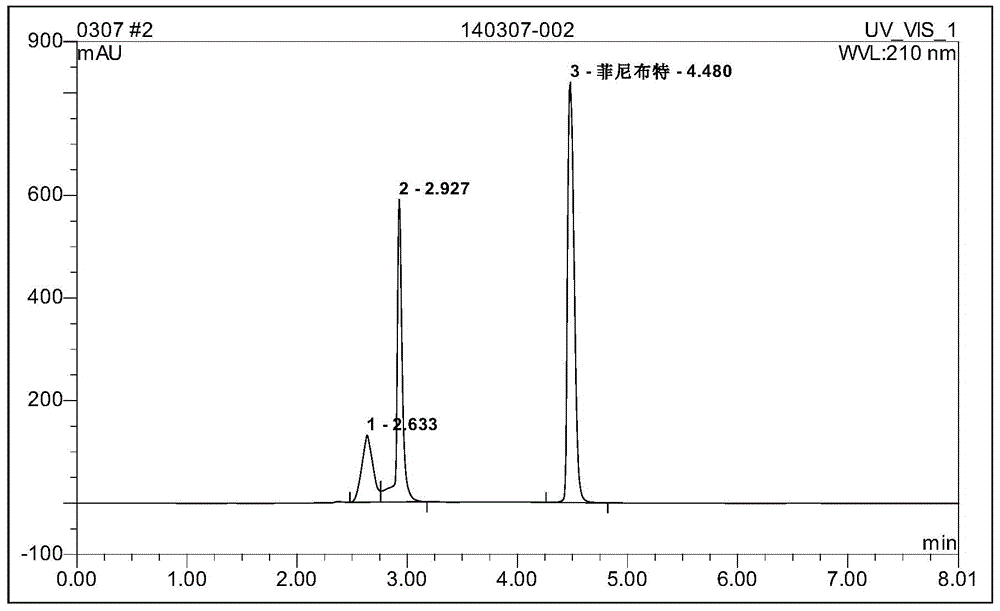
Image
Examples
example 1
[0028] 1] Add 250kg of water into a 1000L reactor, and control the temperature in an ice bath below 15°C, then add 70kg of ethyl acetoacetate into the reactor, stir vigorously and control the temperature not to exceed 15°C; while stirring, dropwise add a mass concentration of 40% sodium hydroxide solution and 70kg mass concentration 99% benzoyl chloride solution keep the pH of the reaction system at about 10. After the dropwise addition, let stand at 55°C for 4-5h; then lower the temperature to 25°C, add 100kg of saturated saline, stir slowly for 10-30 hours, then carry out liquid separation treatment, wash the oily matter with water, and dry over anhydrous sodium sulfate , That is ethyl benzoylacetate.
[0029] 2.1] Get nitromethane 62kg and benzaldehyde 110kg methyl alcohol 400kg and join in the 1000L reactor of step 1 and cool to 0 ℃, then add the sodium hydroxide solution 200kg of concentration 40%, react for three hours after adding, add water and The mass concentration ...
example 2
[0036] 1] Add 500kg of water to a 2000L reactor and control the temperature in an ice bath below 15°C, add 140kg of ethyl acetoacetate, stir vigorously, add dropwise sodium hydroxide with a mass concentration of 40%, control the temperature not exceeding 15°C, and start simultaneously 140 kg of benzoyl chloride with a mass concentration of 99% was added dropwise to control the rate of addition of the two, so that the pH of the reaction system was maintained at about 10, and the reaction system was left to stand at 55° C. for 4-5 hours after the drop was completed. Then, at 25°C, 200 kg of saturated saline was added, slowly stirred overnight, reacted for 20 hours, separated, the oil was washed with water, and dried over anhydrous sodium sulfate. That is ethyl benzoyl acetate.
[0037] 2.1] nitromethane 124kg and benzaldehyde 220kg methanol 800kg join in 2000L reaction kettle and cool down to 0 degree, add mass concentration and be 40% sodium hydroxide 200kg, react for three hou...
example 3
[0044]1] Add 750kg of water to the 3000L reactor and control the temperature in an ice bath below 15°C, add 210kg of ethyl acetoacetate, stir vigorously, add dropwise sodium hydroxide with a mass concentration of 40%, control the temperature not to exceed 15°C, and start simultaneously Add 210 kg of benzoyl chloride with a mass concentration of 99% dropwise to control the drop rate of the two, so that the pH of the reaction system is maintained at about 10. Ethyl acetate, after the drop is completed, let stand at 55°C for 4-5h. Then, at 25°C, 300 kg of saturated saline was added, slowly stirred overnight, reacted for 20 hours, separated, the oil was washed with water, and dried over anhydrous sodium sulfate. That is ethyl benzoyl acetate.
[0045] 2.1] Nitromethane 186kg and benzaldehyde 360kg methyl alcohol 1200kg join in 3000L reactor and cool to 0 degree, add mass concentration and be 40% sodium hydroxide 200kg, react for three hours after adding, add water 300kg, add conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com