Liquid chromatography method for determining clavulanic acid related substances in amoxicillin and clavulanic acid potassium pharmaceutical composition
A technology of amoxicillin-clavulanate potassium and clavulanic acid, which is applied in the field of drug impurity analysis and detection, can solve problems such as interference, and achieve the effects of improving accuracy, facilitating product quality control, and simple and easy cost analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Experimental equipment and conditions:
[0065] Agilent high performance liquid chromatography; DAD detector; Waters Atlantis T3, 5μm, 250×4.6mm as the separation column.
[0066] Detection conditions:
[0067] Flow rate: 1.0ml / min; Detection wavelength: 214nm; Column temperature: 30°C; 0.03mol / L ammonium acetate aqueous solution (adjust the pH value to 5.70 with glacial acetic acid)-acetonitrile (80:20), perform linear gradient elution according to Table 1:
[0068] Table 1 Gradient elution program
[0069]
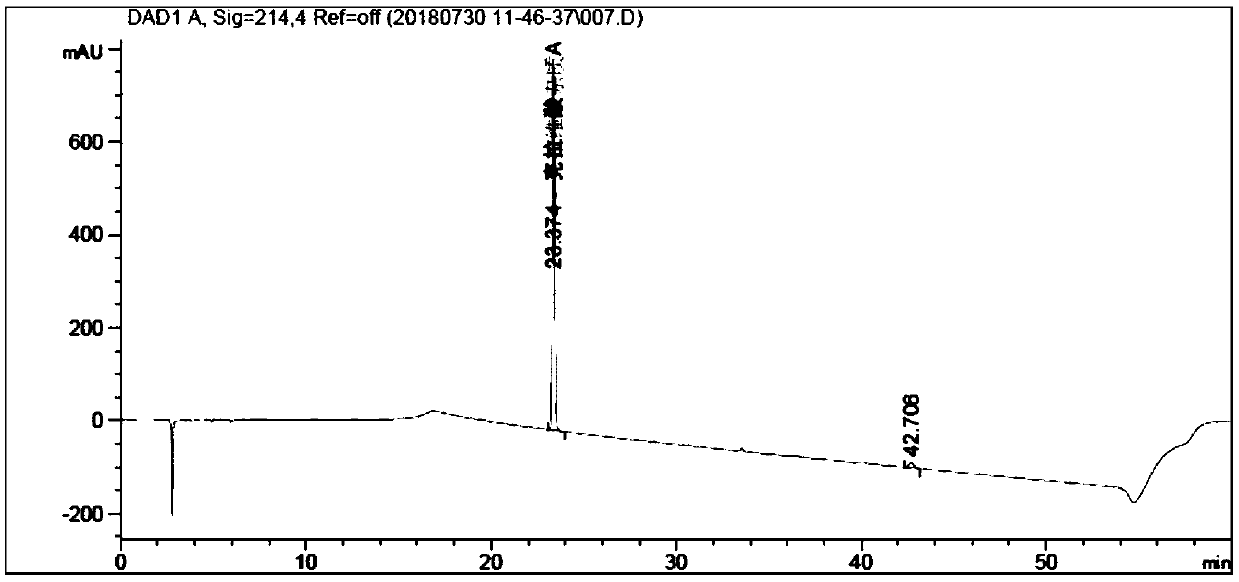
[0070] Experimental procedure: 0.03mol / L ammonium acetate aqueous solution (adjust pH value to 5.70 with glacial acetic acid) as mobile phase A, use 0.03mol / L ammonium acetate aqueous solution (adjust pH value to 5.70 with glacial acetic acid)-acetonitrile (80:20) It is the mobile phase B, eluted according to the gradient in Table 1, the flow rate is 1.0ml / min, the detection wavelength is 214nm, and the column temperature is 30°C; take clavulanic acid impuri...
Embodiment 2
[0073] Experimental equipment and conditions:
[0074] Agilent high performance liquid chromatography; DAD detector; Waters Atlantis T3, 5μm, 250×4.6mm as the separation column.
[0075] Detection conditions:
[0076] Flow rate: 0.8ml / min; Detection wavelength: 214nm; Column temperature: 30°C; 0.05mol / L ammonium acetate aqueous solution (adjust the pH value to 5.70 with glacial acetic acid)-acetonitrile (80:20), perform linear gradient elution according to Table 2:
[0077] Table 2 Gradient elution program
[0078]
[0079]
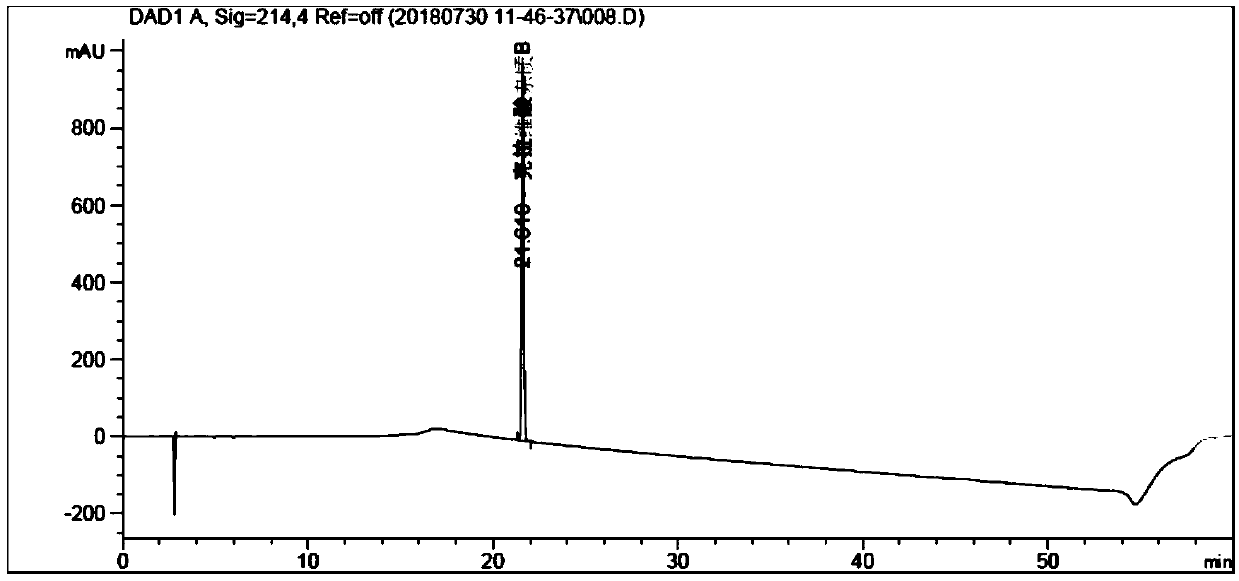
[0080] Experimental procedure: 0.05mol / L ammonium acetate aqueous solution (adjust pH value to 5.70 with glacial acetic acid) as mobile phase A, use 0.05mol / L ammonium acetate aqueous solution (adjust pH value to 5.70 with glacial acetic acid)-acetonitrile (80:20) It is mobile phase B, eluted according to the gradient in Table 2, the flow rate is 0.8ml / min, the detection wavelength is 214nm, and the column temperature is 30°C; take amoxicillin an...
Embodiment 3
[0083] Experimental equipment and conditions:
[0084] Agilent high performance liquid chromatography; DAD detector; Waters Atlantis T3, 5μm, 250×4.6mm as the separation column.
[0085] Detection conditions:
[0086] Flow rate: 1.2ml / min; Detection wavelength: 214nm; Column temperature: 30°C; 0.05mol / L ammonium acetate aqueous solution (adjust the pH value to 5.70 with glacial acetic acid)-acetonitrile (80:20), perform linear gradient elution according to Table 3:
[0087] Table 3 Gradient elution program
[0088]
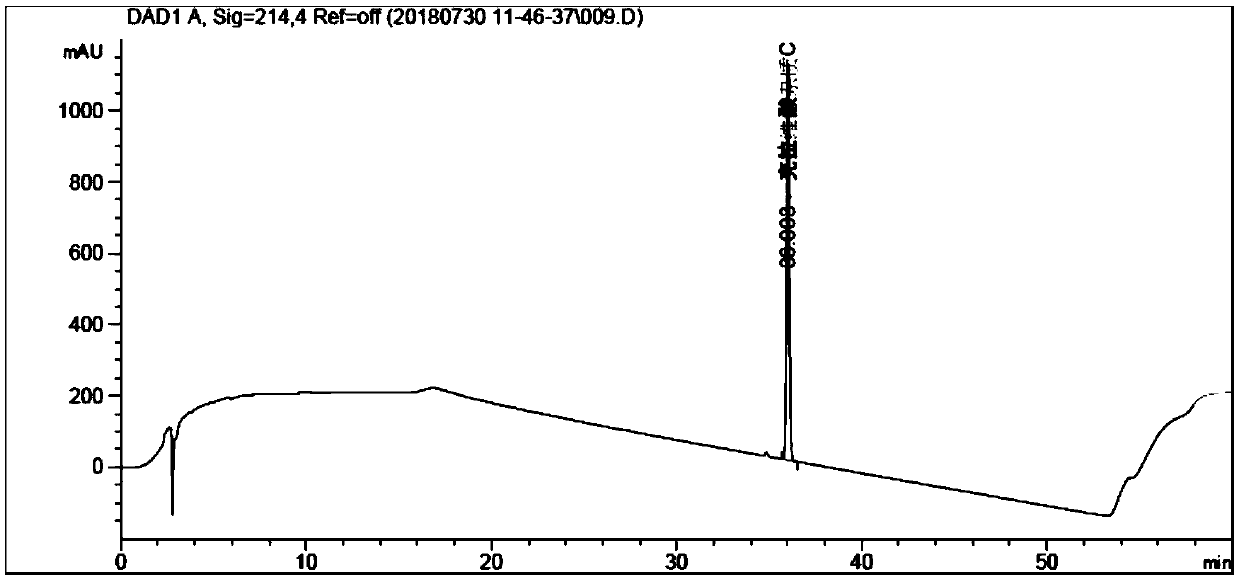
[0089] Experimental procedure: 0.05mol / L ammonium acetate aqueous solution (adjust pH value to 5.70 with glacial acetic acid) as mobile phase A, use 0.05mol / L ammonium acetate aqueous solution (adjust pH value to 5.70 with glacial acetic acid)-acetonitrile (80:20) It is mobile phase B, eluted according to the gradient in Table 3, the flow rate is 1.2ml / min, the detection wavelength is 214nm, and the column temperature is 30°C; take amoxicillin and clavulanat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com