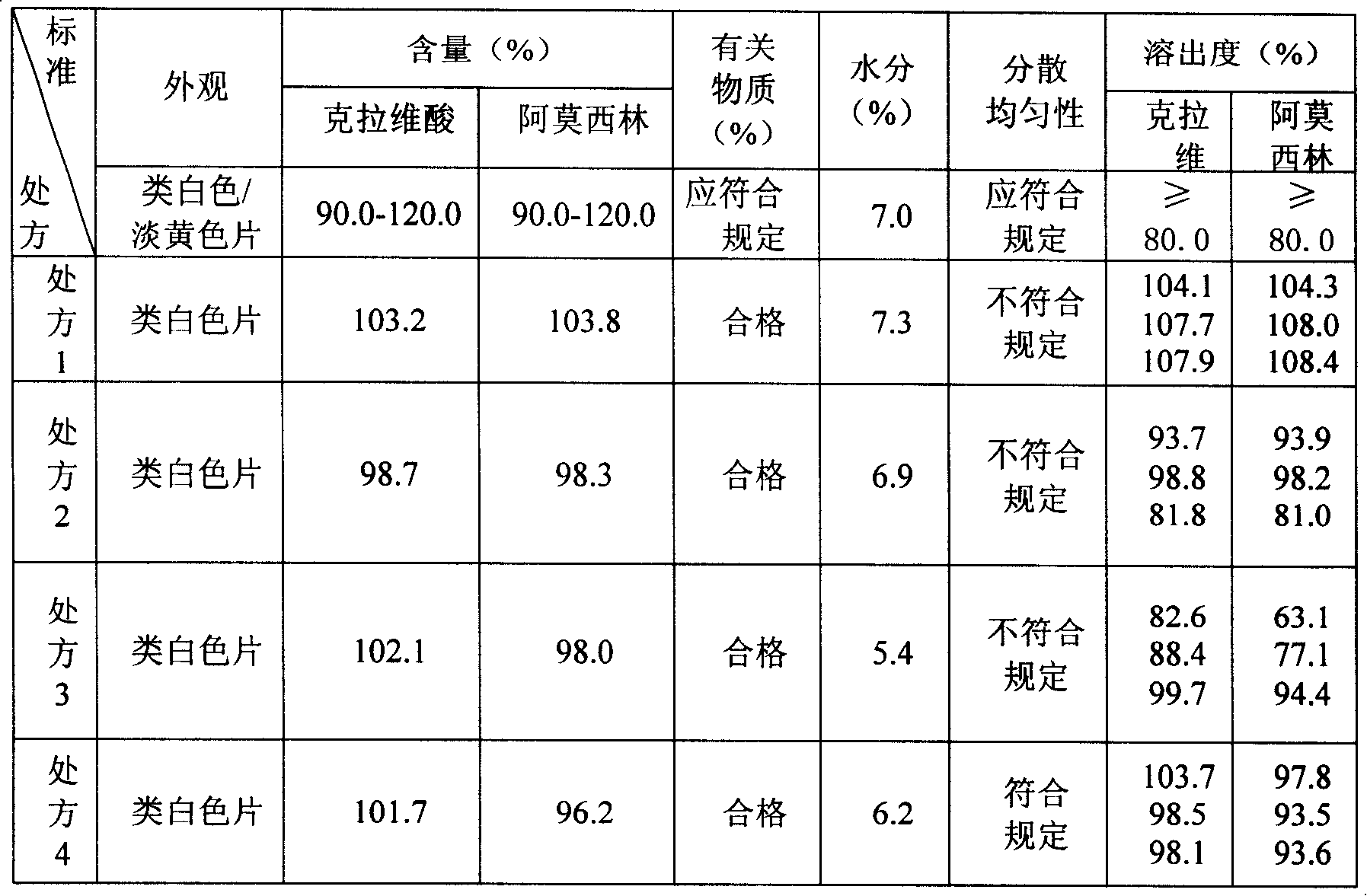
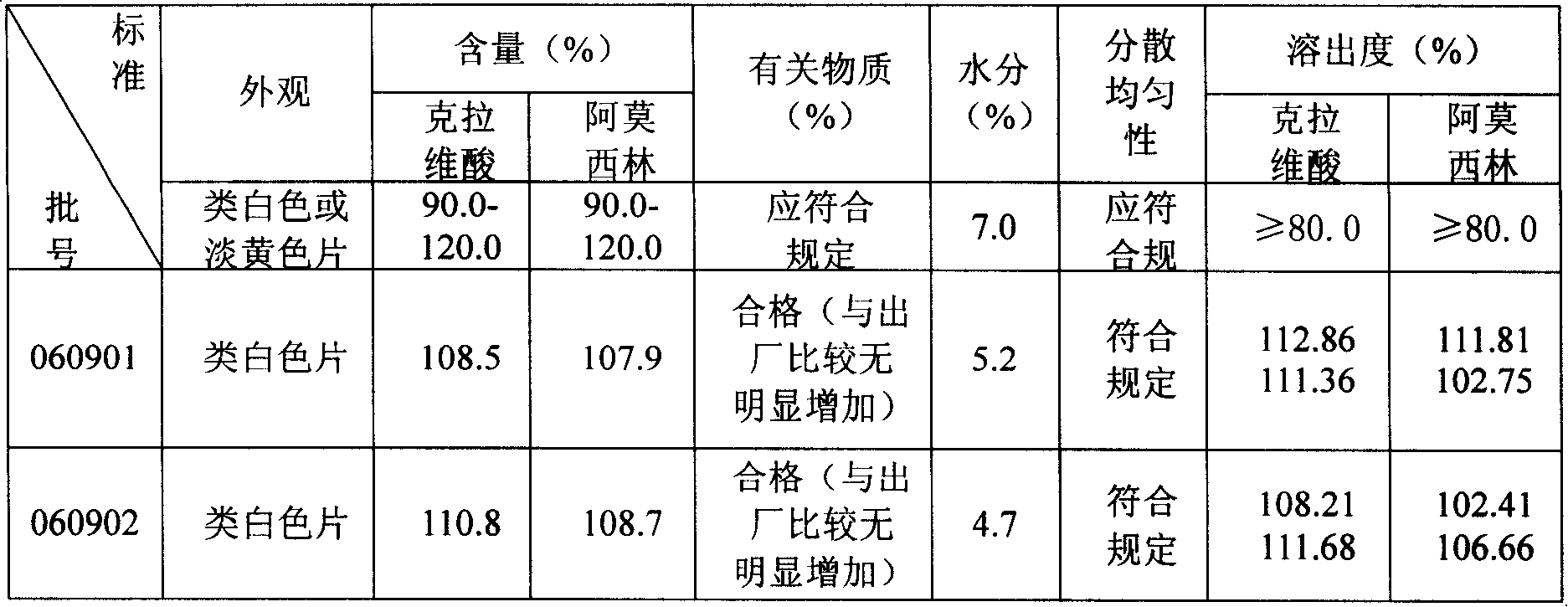
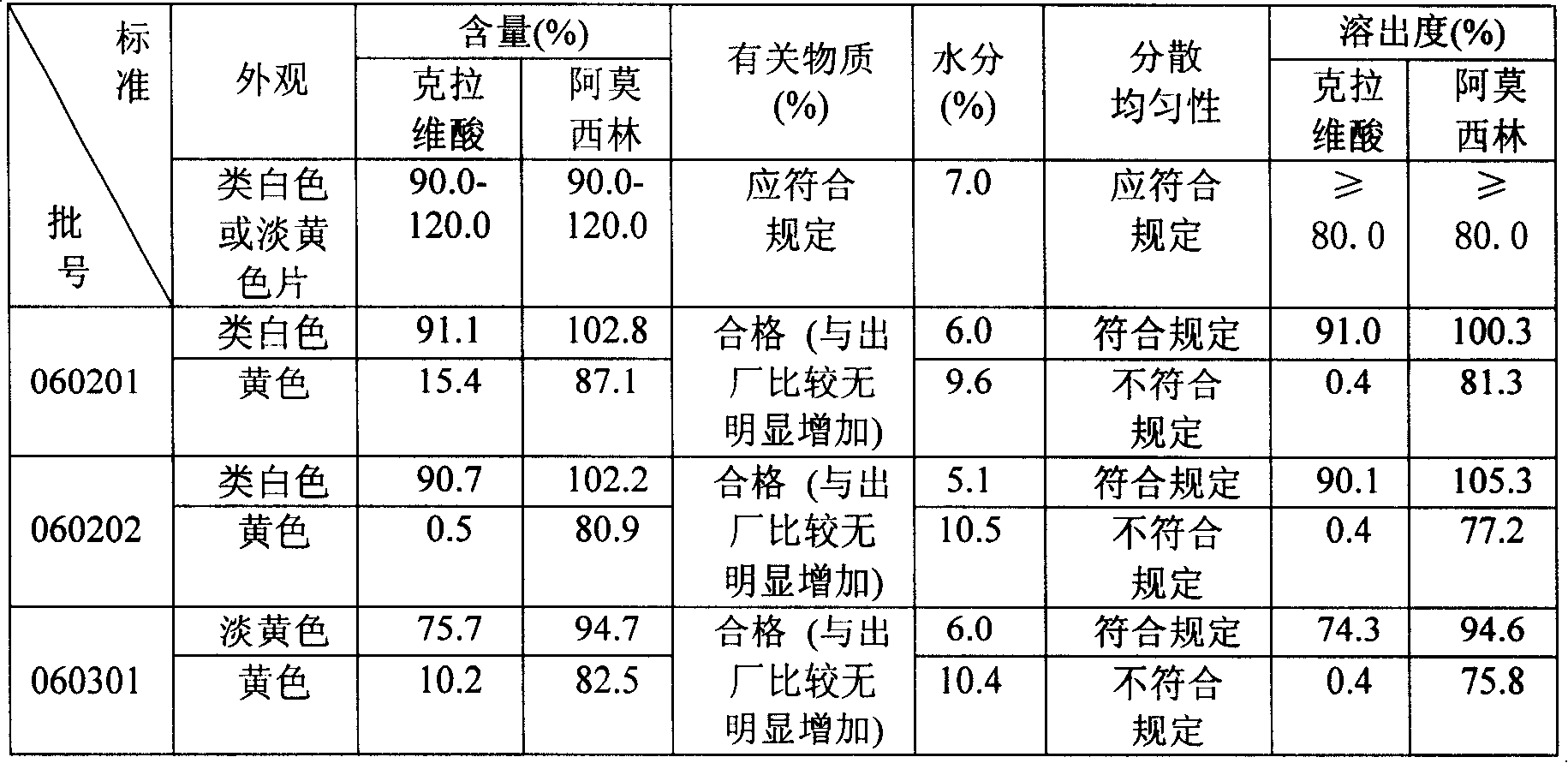
Amoxicillin clavulanate potassium 4:1 dispersible tablet and production technology thereof
A technology of amoxicillin-clavulanate potassium and dispersible tablets, which is applied in the direction of active ingredients of heterocyclic compounds, pill delivery, antibacterial drugs, etc. It can solve the problems that the fluidity is not as good as that of granules, and it is easy to produce capping, so as to achieve fluidity Good resistance, not easy to absorb water, excellent disintegration performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Feeding:
[0044] 1. Potassium Amoxicillin and Clavulanate 4:1 Mixed Powder 156.25g
[0045] 2. Lactose 35g
[0046] 3. Microcrystalline cellulose 65g
[0047] 4. Crystalline Mannitol 170g
[0048] 5. Cross-linked polyvinylpyrrolidone (XL type) 60g
[0049] 6. Micropowder silica gel (solid phase) 10.4g
[0050] 7. Magnesium stearate 5.2g
[0051] 8. Powdered mint essence 6.2g
[0052] 9. Aspartame 3.4g
[0053]
[0054] Total weight 511.45g
[0055] a) Dry lactose, microcrystalline cellulose, crystalline mannitol, cross-linked PVP, and micropowder silica gel at 90°C for 14 hours;
[0056]b) Mix the full amount of lactose, crystalline mannitol, cross-linked PVP, micropowder silica gel and 48g of microcrystalline cellulose according to the above prescription, and put them into the mixer together with the main ingredients and mix for 30 minutes to control the moisture content of the mixed powder < 3.0%;
[0...
Embodiment 2
[0063] Feeding:
[0064] 1. Potassium Amoxicillin and Clavulanate 4:1 Mixed Powder 160g
[0065] 2. Lactose 45g
[0066] 3. Microcrystalline cellulose 68g
[0067] 4. Crystalline Mannitol 183g
[0068] 5. Cross-linked polyvinylpyrrolidone (XL type) 62.5g
[0069] 6. Micropowder silica gel (solid phase) 11.8g
[0070] 7. Magnesium stearate 7.3g
[0071] 8. Powdered peppermint essence 11.6g
[0072] 9. Aspartame 6.9g
[0073]
[0074] Total weight 556.1g
[0075] a) Dry lactose, microcrystalline cellulose, crystalline mannitol, cross-linked PVP, and micronized silica gel at 95° C. for 12 hours;
[0076] b) Mix the full amount of lactose, crystalline mannitol, cross-linked PVP, micropowder silica gel and 35g of microcrystalline cellulose according to the above prescription, and put them into the mixer together with the main ingredients and mix for 40 minutes to control the moisture content of the mixed powder < 3.0%;
...
Embodiment 3
[0083] Feeding:
[0084] 1. Potassium Amoxicillin and Clavulanate 4:1 Mixed Powder 142g
[0085] 2. Lactose 30g
[0086] 3. Microcrystalline cellulose 52g
[0087] 4. Crystalline Mannitol 168g
[0088] 5. Cross-linked polyvinylpyrrolidone (XL type) 53.5g
[0089] 6. Micropowder silica gel (solid phase) 7.8g
[0090] 7. Magnesium stearate 4.2g
[0091] 8. Powdered peppermint essence 7.8g
[0092] 9. Aspartame 3.6g
[0093]
[0094] Total weight 468.9g
[0095] The production process and production environment are the same as in Example 1, wherein the added amount of microcrystalline cellulose is 36g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com