Pharmaceutical formulations comprising amoxicillin and clavulanante
A technology of clavulanate and amoxicillin, which is applied in the field of pharmaceutical preparations containing amoxicillin and clavulanate, and can solve problems such as no beneficial effects observed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 5
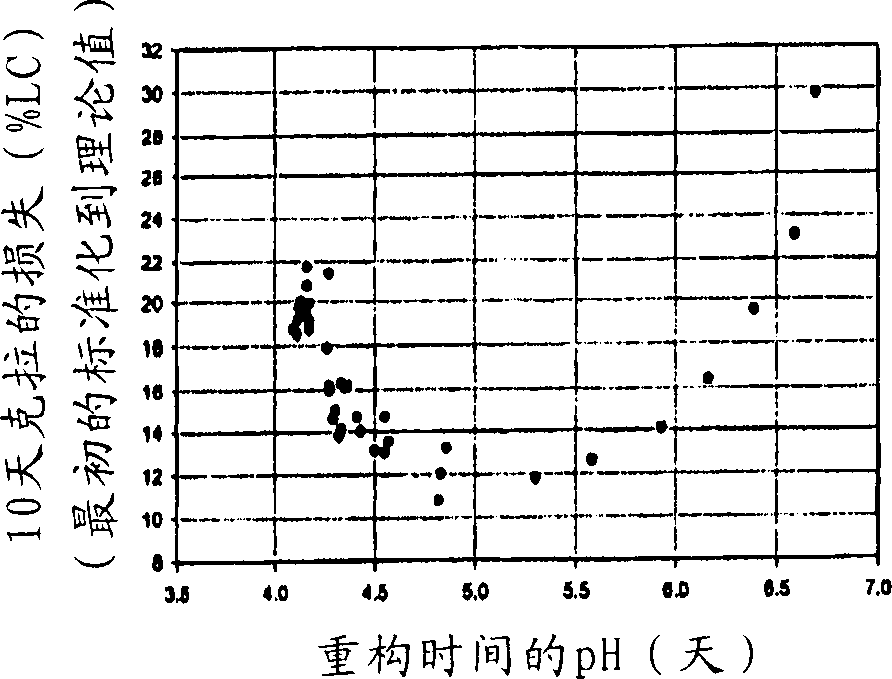
[0071] The effect of adding carboxymethylcellulose sodium to a series of typical formulations containing amoxicillin and clavulanate on the loss of clavulanate from the reconstituted suspension was determined over a period of 10 days. The prototype formulations evaluated corresponded to those in Examples 1A and 2 to 4, with 600 to 1200 mg / 5 ml amoxicillin and 43 mg / 5 ml constant levels of clavulanate. In addition, another formulation with a similar amount of excipient and containing 400 mg / 5 ml, and a reference formulation containing 200 mg / 5 ml were also included for comparison.
[0072] The formulations are made up of defined concentrations with water. Samples were taken initially and after 10 days of storage (at 4°C). Samples were analyzed for clavulanate concentration using HPLC and UV detection. In addition, the pH of the suspension was measured at the same time point.
[0073] The results of the pH change and loss of clavulanate after 10 days are shown in the table be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com