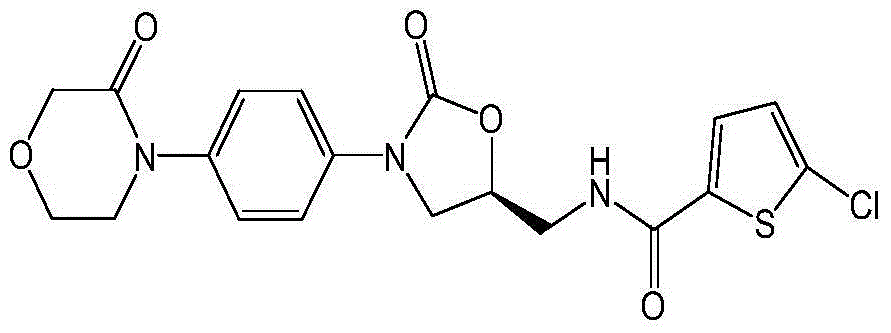
Preparation method of oral preparation containing rivaroxaban
A rivaroxaban and content technology, applied in the field of oral tablets containing rivaroxaban, can solve problems such as sticky punching, complicated preparation process, and loose tablets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
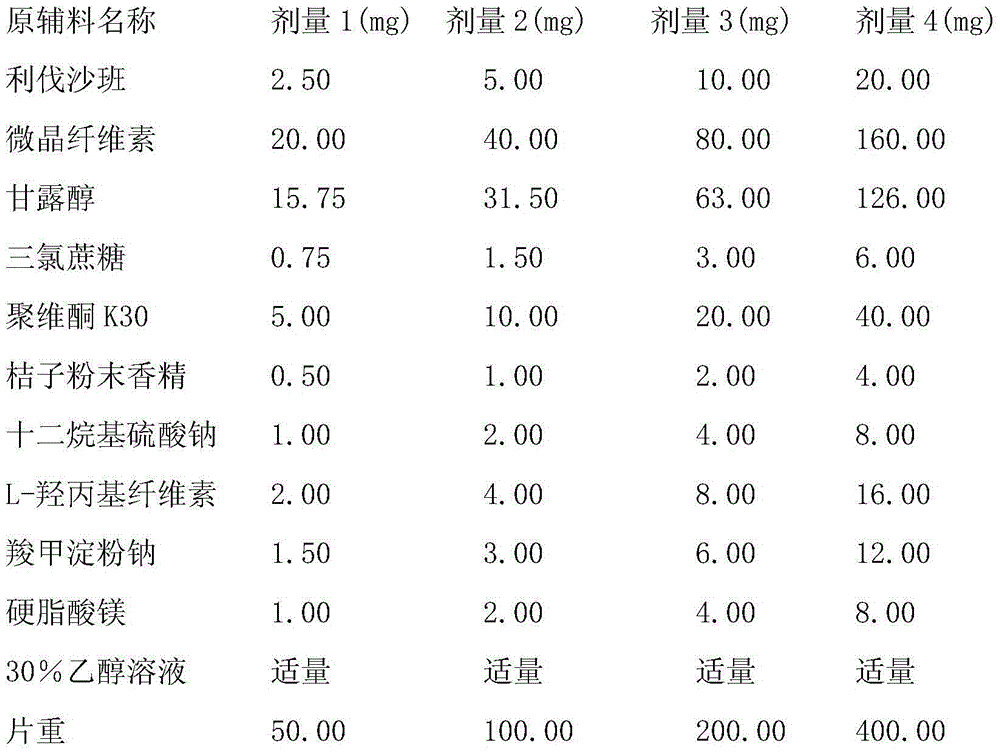
[0053] 10mg tablet
[0054]
[0055] The preparation method is as follows:
[0056] 1. Mix the raw materials with sodium lauryl sulfate evenly - mix for not less than 30 minutes to obtain mixture I;
[0057] 2. Mix mixture Ⅰ and lactose in equal amounts and mix evenly—each time mixing for no less than 20 minutes to obtain mixture Ⅱ;
[0058] 3. Mix the mixture II and the remaining materials evenly, and press into tablets;
Embodiment 2
[0060] 5mg tablet
[0061]
[0062]
[0063] The preparation method is as follows:
[0064] 1. Mix the raw materials with sodium lauryl sulfate evenly - mix for not less than 30 minutes to obtain mixture I;
[0065] 2. Mix mixture Ⅰ and lactose in equal amounts and mix evenly—each time mixing for no less than 20 minutes to obtain mixture Ⅱ;
[0066] 3. Mix the mixture II and the remaining materials evenly, and press into tablets;
Embodiment 3
[0068] 20mg tablet
[0069]
[0070] The preparation method is as follows:
[0071] 1. Mix the raw materials with sodium lauryl sulfate evenly - mix for not less than 30 minutes to obtain mixture I;
[0072] 2. Mix mixture Ⅰ and lactose in equal amounts and mix evenly—each time mixing for no less than 20 minutes to obtain mixture Ⅱ;
[0073] 3. Mix the mixture II and the remaining materials evenly, and press into tablets;
[0074] In the above embodiments, if 100,000 pieces are prepared in industrial scale-up production, the weight of the raw and auxiliary materials will increase by 100,000 times accordingly.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com