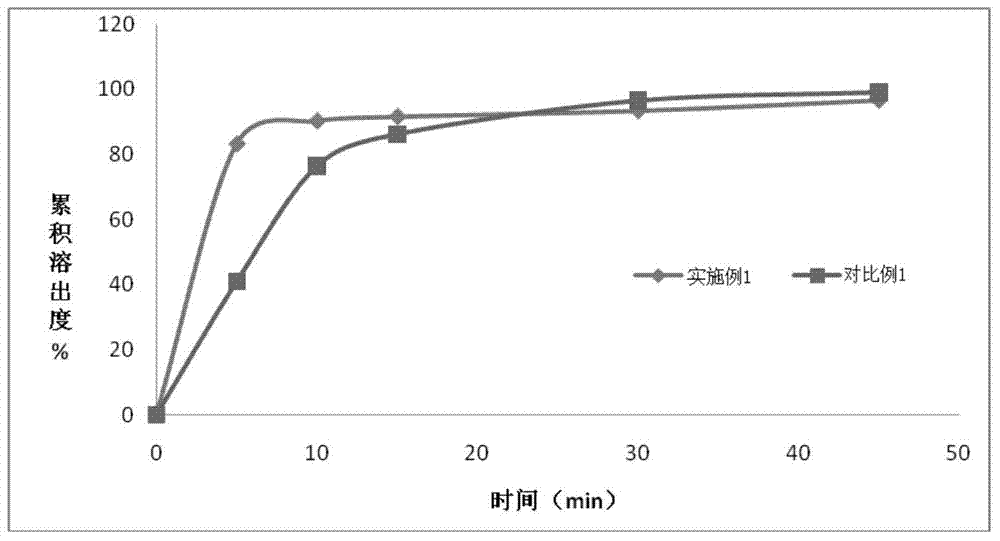
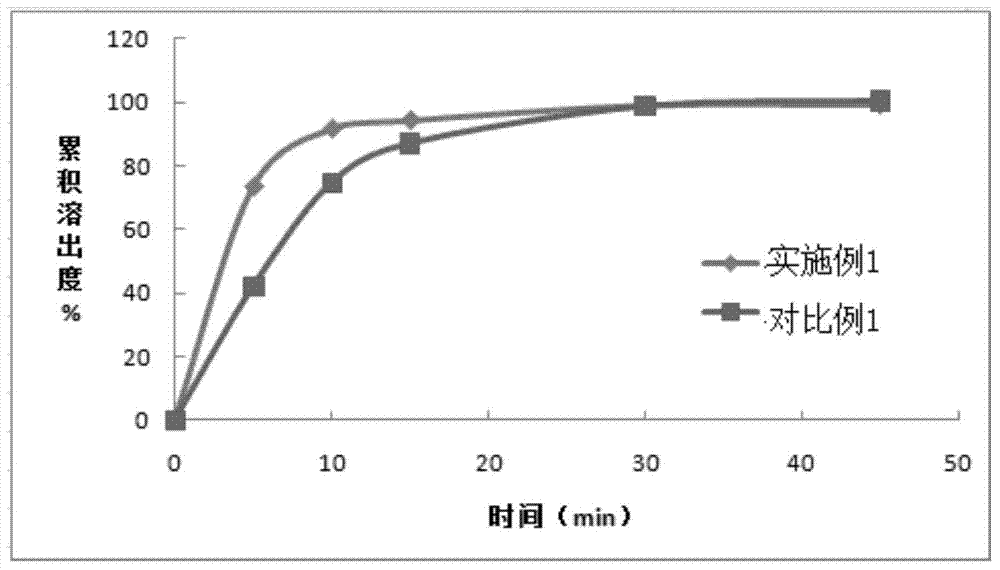
Apixaban tablet and preparation method thereof
A technology for apixaban tablets and tablet cores, applied in the field of medicine, can solve the problems of high impurity content and slow dissolution rate, and achieve the effects of simple technological process, low impurity content, and improved dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The apixaban tablet in this example includes a tablet core and a coating. The tablet core consists of the following components in percentage by weight: 2.6% of apixaban, 92.3% of lactose-cellulose complex, croscarmellose Sodium cellulose 3.1%, sodium lauryl sulfate 1.0%, magnesium stearate 1.0%;
[0052] The coating is made of the following components in mass percentage: hypromellose E56.01%, red iron oxide 0.1%, titanium dioxide 3.99%, and water 89.9%.
[0053] In this example, 1000 apixaban tablets were prepared, each apixaban tablet contained 5 mg of apixaban, and the coating weight was 2.6% to 5.4% of the tablet core weight.
[0054] The preparation method of the Apixaban tablet in this embodiment is, and the specific operation steps are:
[0055] 1) Micronizing 5.0 g of apixaban, controlling the particle size to be d90<10.0 μm, d50<4.0 μm, and d10<3.0 μm, to obtain apixaban micropowder;
[0056] 2) Premix the apixaban micropowder prepared in step 1) with 180.0 g ...
Embodiment 2
[0061] The apixaban tablet in this example includes a tablet core and a coating. The tablet core consists of the following components in percentage by weight: 2.6% of apixaban, 92.3% of lactose-cellulose complex, croscarmellose Sodium cellulose 3.1%, sodium lauryl sulfate 1.0%, magnesium stearate 1.0%;
[0062] The coating is made of the following components in mass percentage: hypromellose E56.01%, red iron oxide 0.1%, titanium dioxide 3.99%, and water 89.9%.
[0063] In this example, 1000 apixaban tablets were prepared, each containing 2.5 mg of apixaban, and the weight of the coating was 2.6% to 5.4% of the weight of the tablet core.
[0064] The preparation method of the Apixaban tablet in this embodiment is, and the specific operation steps are:
[0065] 1) Micronizing 2.5 g of apixaban, controlling the particle size to be d90<10.0 μm, d50<4.0 μm, and d10<3.0 μm, to obtain apixaban micropowder;
[0066] 2) Premix the apixaban micropowder prepared in step 1) with 90.0 g ...
Embodiment 3
[0071] The apixaban tablet in this example includes a tablet core and a coating, and the tablet core is composed of the following components in percentage by weight: 2.6% of apixaban, 92.1% of lactose-cellulose complex, croscarmellose Sodium cellulose 3.1%, sodium lauryl sulfate 1.1%, magnesium stearate 1.1%;
[0072] The coating is made of the following components in mass percentage: hypromellose E56.01%, red iron oxide 0.1%, titanium dioxide 3.99%, and water 89.9%.
[0073] In this example, 1000 apixaban tablets were prepared, each containing 2.5 mg of apixaban, and the weight of the coating was 2.6% to 5.4% of the weight of the tablet core.
[0074] The preparation method of the Apixaban tablet in this embodiment is, and the specific operation steps are:
[0075] 1) Micronize 2.5g of apixaban to control the particle size to d 90 50 10 <3.0μm, get apixaban micropowder;
[0076] 2) Premix the apixaban micropowder prepared in step 1) with 87.5 g of croscarmellose sodium, 3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com