Divalproex sodium sustained release tablet
A technology of sodium divalproex and sustained-release tablets, which is applied in the directions of anhydride/acid/halide active ingredients, medical preparations of non-active ingredients, and pill delivery, etc., can solve problems such as increase in tablet weight and related substances, and achieve The effect of uniform sample content, avoiding large fluctuations and ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Preparation of divalproex sodium granules by two granulation processes
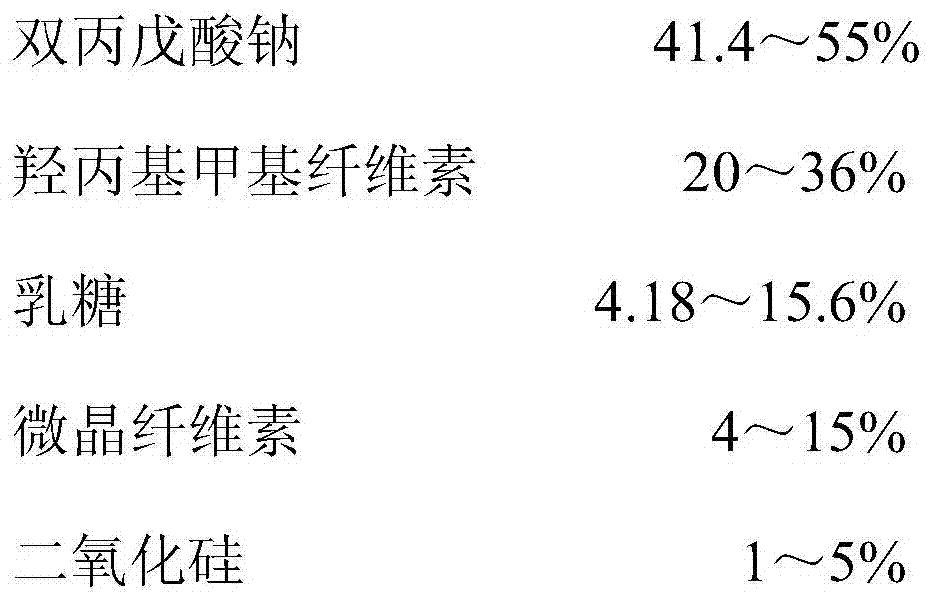
[0043] 1. The weight percentage of each component
[0044]
[0045] 2. Experimental method
[0046] (1) process granulation method of the present invention prepares divalproex sodium granules
[0047] a) Pulverize divalproex sodium and pass through a 100-mesh sieve;
[0048] b) mixing hydroxypropyl methylcellulose, microcrystalline cellulose and lactose to obtain a uniformly dispersed mixed material;
[0049] c) Adding an appropriate amount of ethanol to the homogeneously mixed material prepared in step b) under the stirring condition of the wet mixing granulator, the amount of ethanol added is 150-300 mL per 1 kg of material to obtain a wet and soft material;
[0050] d) Add the divalproex sodium prepared in step a) to the wet soft material prepared in step c) under the stirring condition of the wet mixing granulator, and continue to mix the materials with the wet mixing granulator, ...
Embodiment 2
[0074] prescription:
[0075]
[0076]
[0077] Process: prepare wet granules according to the process in Example 1, dry the wet granules at 50°C for 1 hour, sieve the granules with a 24 mesh sieve, mix them evenly with 3.0% silicon dioxide by weight, and press them into tablets. The die size is 1.9 cm ×0.91cm (oval shape), tablet weight 1000mg, pressure 130-150N.
[0078] Get the dried granules prepared in the above process, adopt the sieving rate method in Example 1 to measure the sieving rate by 24 mesh sieves, the result is 99.24%. Get each 6 of divalproex sodium sustained-release tablets made above, adopt the method in embodiment 1 to measure the content of divalproex sodium in every tablet, the results are as follows:
[0079]
Embodiment 3
[0081] prescription:
[0082]
[0083] Process: Prepare wet granules according to the process in Example 1, dry the wet granules at 60°C for 1 hour, sieve the granules with a 20-mesh sieve, mix them evenly with 2.0% silicon dioxide by weight, and press them into tablets. The die size is 1.9 cm ×0.91cm (oval shape), tablet weight 1000mg, pressure 120-135N.
[0084] Get the dried granules prepared in the above process, adopt the sieving rate method in Example 1 to measure the sieving rate by 20 mesh sieves, and the result is 100%. Get each 6 of divalproex sodium sustained-release tablets made above, adopt the method in embodiment 1 to measure the content of divalproex sodium in every tablet, the results are as follows:
[0085]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com