Divalproex sodium sustained release tablets and preparation method thereof
A technology of sodium divalproex and sustained-release tablets, which can be applied in pharmaceutical formulations, medical preparations with inactive ingredients, anhydride/acid/halide active ingredients, etc., and can solve the volume of oral sustained-release preparations of sodium divalproex. It is difficult to swallow and other problems, and achieves the effect of reducing internal and external differences, the process is simple, and the process is easy to implement.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
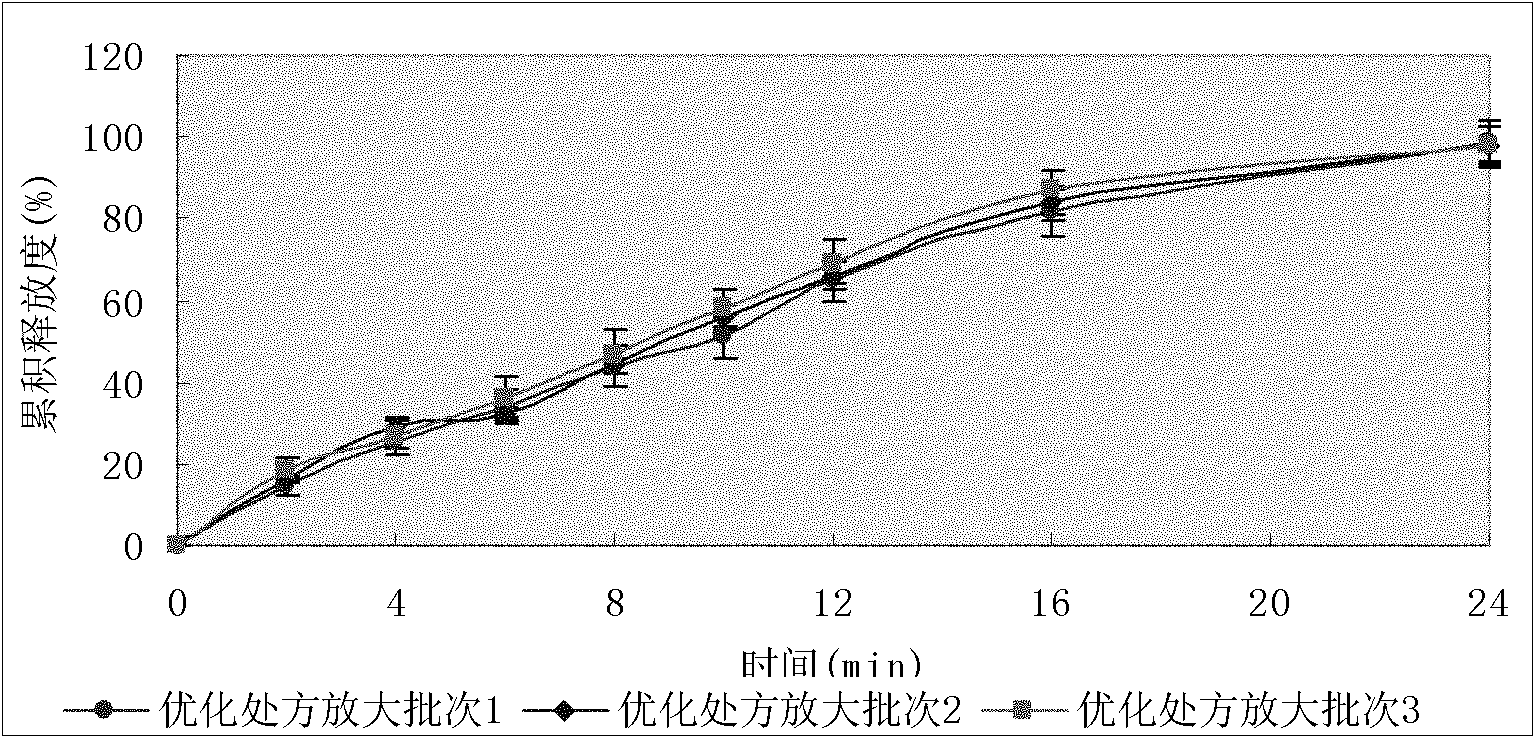
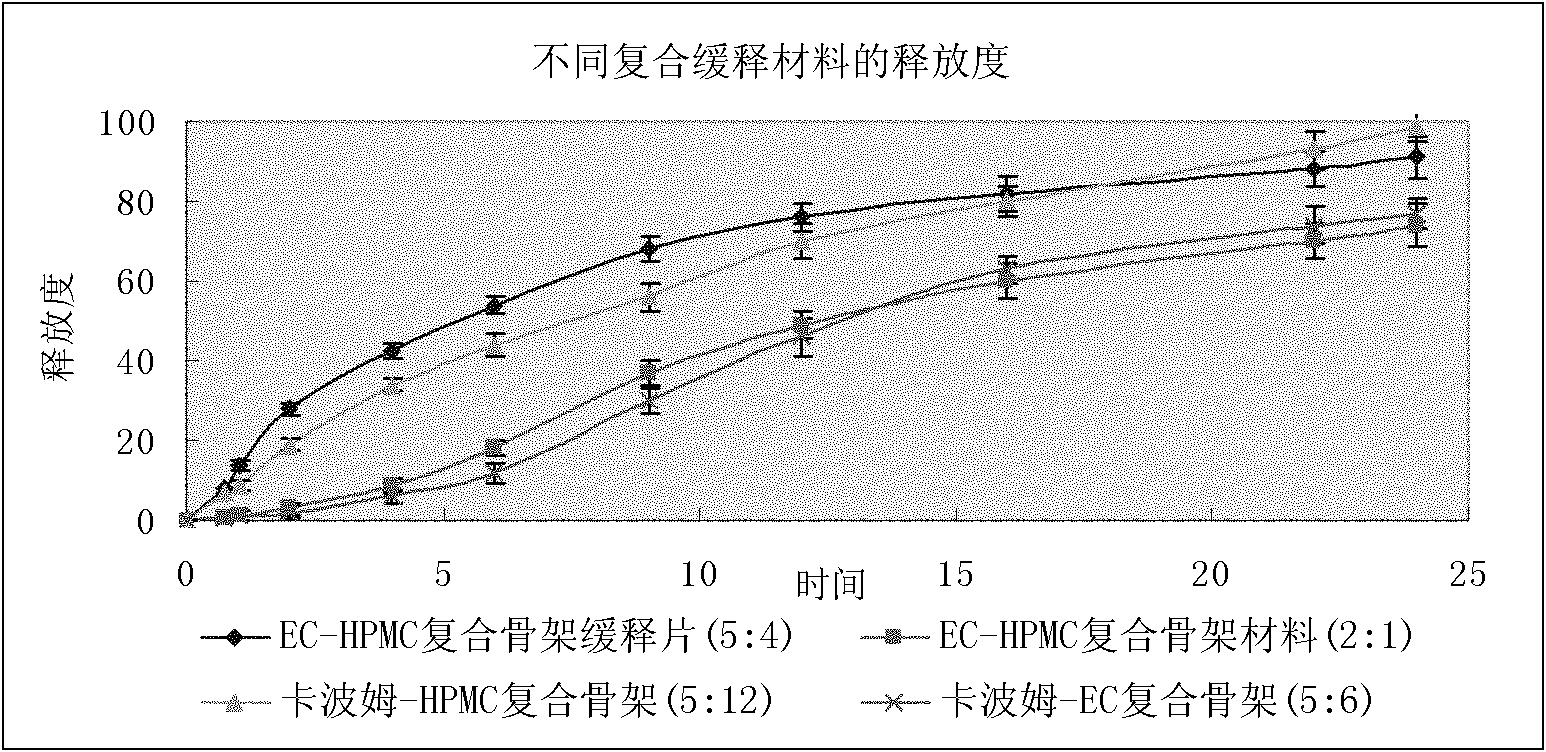
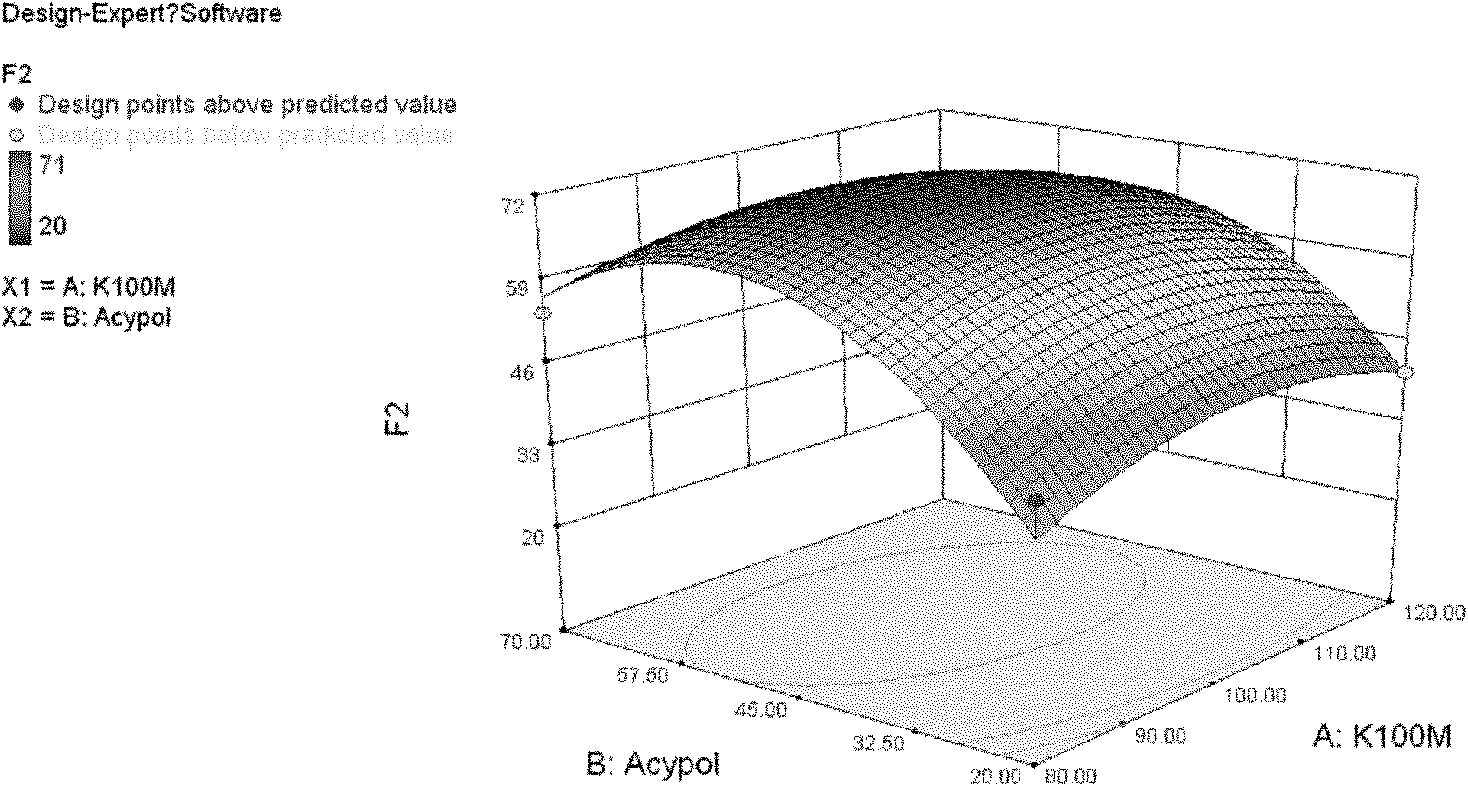
Image
Examples
Embodiment 1
[0060] Prescription for 1000 divalproex sodium extended-release tablets
[0061]
[0062]
[0063] Preparation process and process (oscillating granulator granulation method):
[0064] Take the prescribed amount of medicine, microcrystalline cellulose, hydroxypropyl cellulose, carbomer, Starch, and calcium hydrogen phosphate, pass through an 80-mesh sieve, mix in a mixer for 1 hour, and set aside. Take an appropriate amount of 15% povidone solution, spray it into the standby powder and stir to make a soft material. Turn on the oscillating granulator, pass the soft material through a 24-mesh screen, extrude to form granules of suitable length, place in an oven to dry at 70 degrees Celsius for 60 minutes, check the uniformity and water content, and add the prescribed amount of stearin after passing the test Divalproex sodium and micropowder silica gel are mixed for 1 hour and then added to a tablet press for tableting to obtain divalproex sodium sustained-release tablets....
Embodiment 2
[0066] Prescription for 1000 divalproex sodium extended-release tablets
[0067]
[0068] Appropriate amount of 8% low-viscosity hydroxypropylmethylcellulose aqueous solution
[0069] Preparation process and process:
[0070] The medicine is pulverized with the prescribed amount of medicine, microcrystalline cellulose, hydroxypropyl cellulose, carbomer, Starch, and calcium hydrogen phosphate, and after passing through an 80-mesh sieve, mix it in a stirring mixer for 1 hour, and then mix the powder placed in a fluidized bed. Configure 8% low-viscosity hydroxypropyl methylcellulose aqueous solution, add the prescribed amount of micropowder silica gel and stir to make it into a suspension, and use a fluidized bed for granulation. The fluidized bed granulation process is: peristaltic pump infusion speed is 3ml / min, the pressure of the air compressor is 2Bar, and the air inlet temperature of the fluidized bed is 35°C; after a period of fluidization, take particles with a part...
Embodiment 3
[0072] Prescription for 1000 divalproex sodium extended-release tablets
[0073]
[0074] Preparation Process:
[0075] Preparation process of sustained-release tablet core:
[0076] The medicine is pulverized with the prescribed amount of medicine, microcrystalline cellulose, hydroxypropyl cellulose, lactose, carbomer, Starch, and calcium hydrogen phosphate, and mixed in a stirring mixer for 1 hour after passing through an 80-mesh sieve. Add the magnesium stearate and the talcum powder of recipe quantity, add in the tablet press machine after mixing 0.5 hour in the mixer and carry out direct tableting, both get slow-release tablet.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com