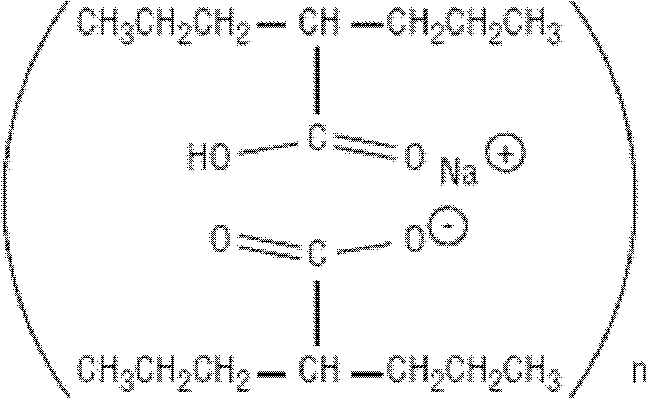Valproate semisodium tablet and its preparation method
A technology of semi-sodium valproate and coated tablets, which is applied in the fields of pill delivery, pharmaceutical formulations, and medical preparations of non-active ingredients. It can solve the problems of unqualified tablet appearance and poor particle fluidity, and achieve appearance maintenance. Intact, stable content, stable quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
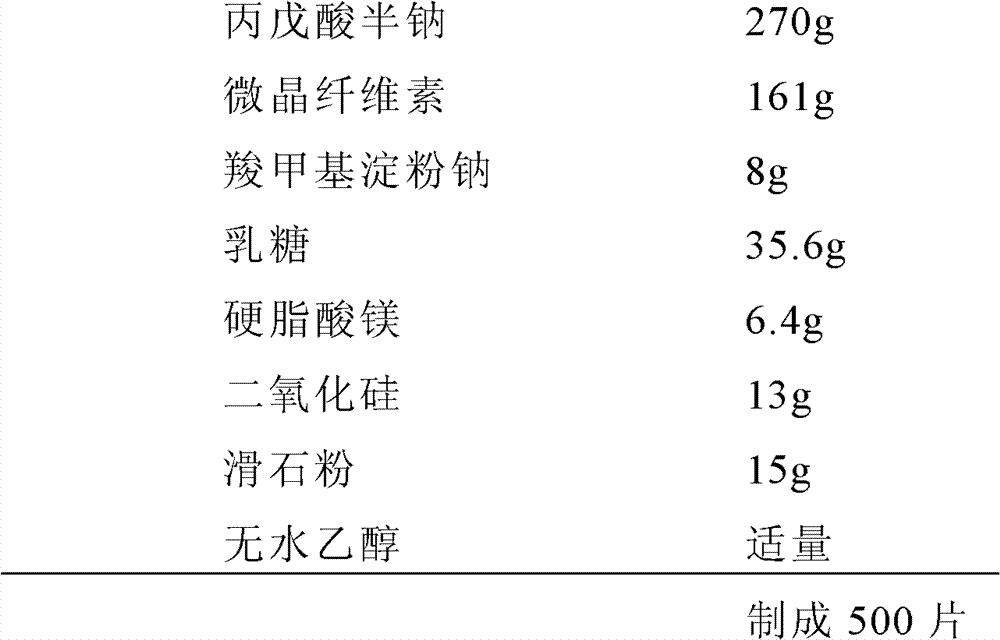
[0082] Example 1 (Valproic acid half-sodium ordinary tablets, 500mg / tablet based on valproic acid)
[0083] prescription:
[0084]
[0085] Preparation:
[0086] Weigh 270g semi-sodium valproate, 161g microcrystalline cellulose, 3.2g sodium carboxymethyl starch, and 35.6g lactose, respectively pass through a 100-mesh sieve, and mix them; use absolute ethanol as a wetting agent to prepare the above-mentioned mixed fine powder Become a soft material, pass a 20-mesh sieve to make granules, dry, sizing, and dry; weigh the remaining 4.8 g of sodium carboxymethyl starch, 6.4 g of magnesium stearate, 15 g of talcum powder, and 13 g of silicon dioxide into the above granules In the middle, mix well; compress tablets at a rotation speed of 25-50r / min.
[0087] Friability check
[0088] According to the requirements of Appendix XG of the Second Part of Chinese Pharmacopoeia 2005, the friability of the tablets of Example 1 was checked, and the weight loss was 0.31%, and there were no broken, cra...
Embodiment 2
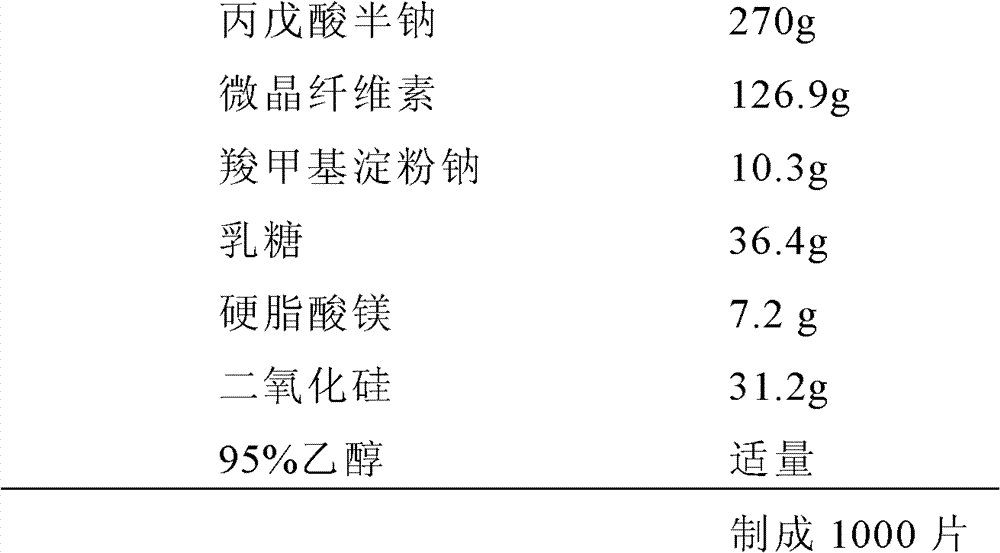
[0089] Example 2 (Valproic acid half-sodium ordinary tablets, 250mg / tablet based on valproic acid)
[0090] prescription:
[0091]
[0092] Preparation:
[0093] Weigh 270g semi-sodium valproate, 126.9g microcrystalline cellulose, 4.6g sodium carboxymethyl starch, and 36.4g lactose, respectively pass through a 100-mesh sieve, and mix them; use 95% ethanol aqueous solution as a wetting agent to mix the above The powder is made into soft material, passed through a 20-mesh sieve to make granules, dried, granulated, and dried; weigh the remaining 5.7g of sodium carboxymethyl starch, 7.2g of magnesium stearate and 31.2g of silicon dioxide and add them to the above granules , Mix well; compress tablets at a speed of 25-50r / min.
[0094] Friability check
[0095] According to the requirements of Appendix XG of the Second Part of Chinese Pharmacopoeia 2005, the friability of the tablets of Example 2 was checked, the weight loss was 0.28%, and there were no broken, cracked or crushed tablets. ...
Embodiment 3
[0096] Example 3 (Semi-sodium valproic acid enteric-coated tablets, 250 mg / tablet based on valproic acid)
[0097] prescription:
[0098]
[0099] Preparation:
[0100] (A) Preparation of tablet core
[0101] Weigh 270g semi-sodium valproate and 155g microcrystalline cellulose, 3.8g sodium carboxymethyl starch, and 34.5g lactose, respectively pass through a 100-mesh sieve, and mix them; use 95% ethanol aqueous solution as a wetting agent to mix the above fine powder Make soft material, sieved through 20 mesh to make granules, dried, sized and dried; weigh the remaining amount of sodium carboxymethyl starch 4.7g, magnesium stearate 7g, and silicon dioxide 30g into the above granules and mix well ; Compress tablets at a speed of 25-50r / min;
[0102] (B) Preparation of enteric-coated tablets
[0103] The 1000 tablet cores obtained in (A) above were fluidized in a fluidized bed coating device and sprayed with OY-P-7171 to obtain semi-sodium valproate enteric-coated tablets (the weight gain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com