Preparation method of progestin sustained-release gel for treating threatened abortion
A technology for slow-release gel and progesterone, which can be applied to medical preparations without active ingredients, medical preparations containing active ingredients, sexual diseases, etc., and can solve problems such as drug loss, reduction, and inconvenience of medication that are not considered.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
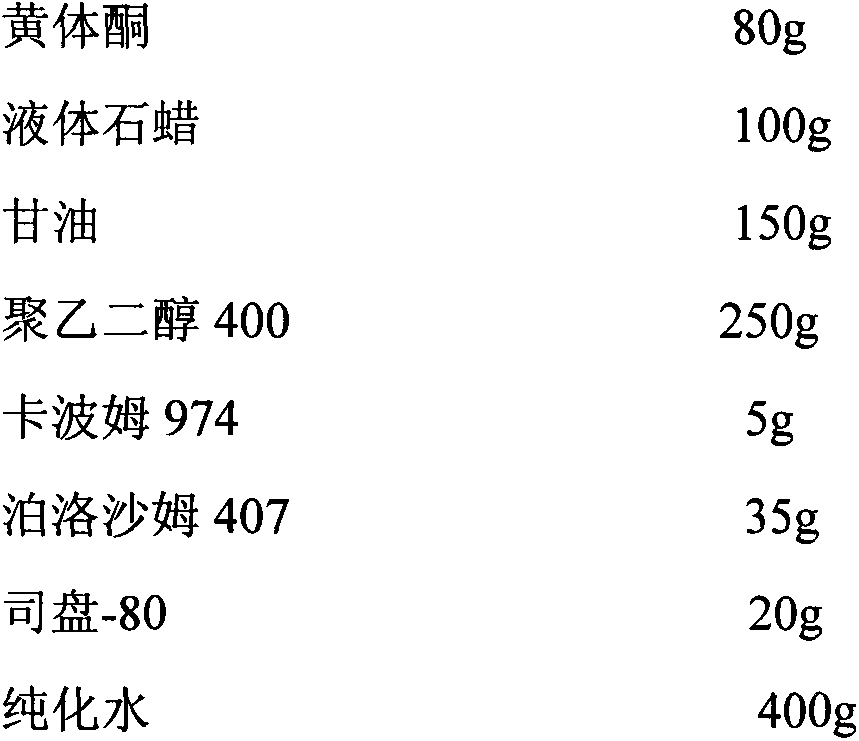
[0025]
[0026] Process:
[0027] Preparation of progesterone emulsion: oil phase, heating and melting liquid paraffin and Span-80, after reaching 70°C, add micronized progesterone and stir. For the water phase, mix polyethylene glycol 400, purified water and glycerin, and heat to 60°C. Add the oil phase to the water phase while stirring continuously and cooling down. Homogenize and form an emulsion.
[0028] Preparation of temperature-sensitive gel: Add Poloxamer 407 to fully swollen Carbomer 974 aqueous solution, stir evenly, add progesterone emulsion into the temperature-sensitive gel matrix, stir continuously, cool down, and adjust with sodium hydroxide solution pH to 4.5, that's it.
Embodiment 2
[0030]
[0031]
[0032] Process:
[0033] Preparation of progesterone emulsion: In the oil phase, liquid paraffin and Tween-80 are heated and melted, and after reaching 70°C, micronized progesterone is added and stirred. For the aqueous phase, mix and dissolve purified water, polyethylene glycol 400, sorbic acid, and glycerin, and heat to 60°C. Add the oil phase to the water phase while stirring continuously to homogenize and form an emulsion.
[0034] Preparation of temperature-sensitive gel: Add poloxamer 407 to fully swollen carbomer 974 aqueous solution, adjust the pH to 4.1 with sodium hydroxide solution, stir evenly, add the emulsion of progesterone into the temperature-sensitive gel matrix, and continuously Stir, cool down, and serve.
Embodiment 3
[0036]
[0037] Process:
[0038] Preparation of progesterone emulsion: In the oil phase, liquid paraffin, hydrogenated palm oil glyceride, and glyceryl monostearate are heated and melted, and after reaching 70°C, micronized progesterone is added and stirred. For the water phase, mix and dissolve polyethylene glycol 400, purified water, and disodium EDTA, and heat to 60°C. The oil phase is added to the water phase while stirring continuously to homogenize and form an emulsion, and the pH is adjusted to 3.5 with sodium hydroxide.
[0039]Preparation of temperature-sensitive gel: Add poloxamer 407 into fully swollen carbomer aqueous solution, stir evenly, add progesterone emulsion into the temperature-sensitive gel matrix, stir continuously, and cool down.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com