Treatment of hereditary angioedema
An angioedema, genetic technology, applied in the field of treatment of hereditary angioedema, can solve problems such as high patient burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
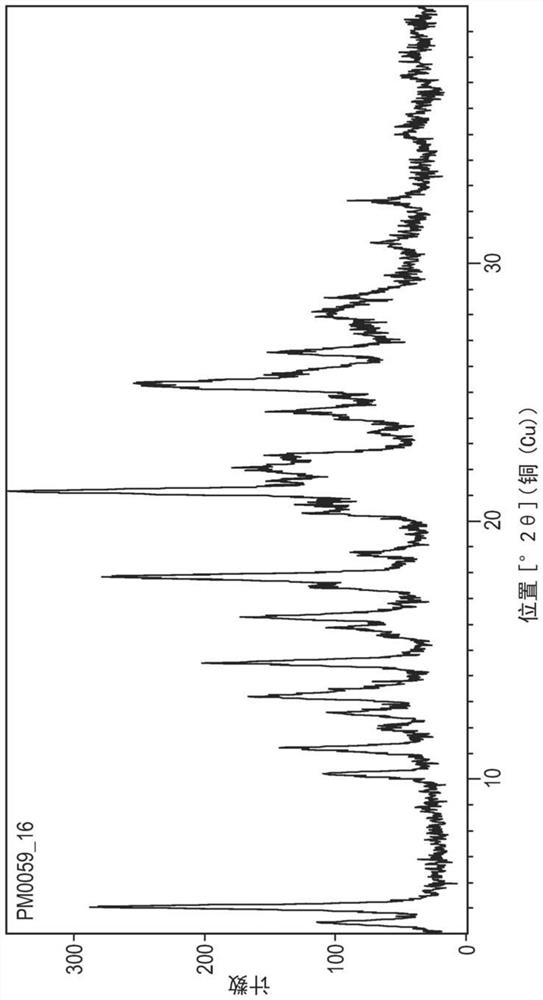
[0154] Example 1 - Preparation of Compounds of Formula A
[0155] A. 1-(4-Hydroxymethyl-benzyl)-1H-pyridin-2-one
[0156] 4-(Chloromethyl)benzyl alcohol (5.0 g, 31.93 mmol) was dissolved in acetone (150 mL). 2-Hydroxypyridine (3.64 g, 38.3 mmol) and potassium carbonate (13.24 g, 95.78 mmol) were added and the reaction mixture was stirred at 50 °C for 3 h, after which the solvent was removed in vacuo and the residue was dissolved in chloroform (100 mL) )middle. The solution was washed with water (30 mL), brine (30 mL) and dried (Na 2 SO 4 ) and evaporated in vacuo. The residue was purified by flash chromatography (silica), eluent 3% MeOH / 97% CHCl 3 , gave a white solid, which was identified as 1-(4-hydroxymethyl-benzyl)-1H-pyridin-2-one (5.30 g, 24.62 mmol, 77% yield).
[0157] [M+Na] + = 238
[0158] B. 1-(4-Chloromethyl-benzyl)-1H-pyridin-2-one
[0159] 1-(4-Hydroxymethyl-benzyl)-1H-pyridin-2-one (8.45 g, 39.3 mmol), dry DCM (80 mL) and triethylamine (7.66 ml, 55.0...
Embodiment 2
[0183] Example 2 - Preparation of Dosage Forms Comprising Compounds of Formula A
[0184] Blending and Rolling
[0185] Equipment: Freund Vector TFC Lab Micro Roller and Pelletizer (Roller and Pelletizer are separate entities). The device parameters are as follows:
[0186] parameter scope of use Screw speed(rpm) 10.0-20.0 Roll speed (rpm) 1.0-2.0 Roll force (kN) 0.50-12.00 Granulator screen size (mm) 1
[0187] method
[0188] Two tablet formulations (tablets A and B) were prepared at the 30 g blending scale according to the following procedure to produce tablets with the components in the amounts indicated below.
[0189]
[0190] For each of the tablets, a blend was prepared by passing the intragranular components through a 355 μm screen in a glass vessel using a Turbula blender at 34 rpm at a scale suitable for the roller compactor range. The blend was then passed through a roller press using the parameters described abo...
Embodiment 3
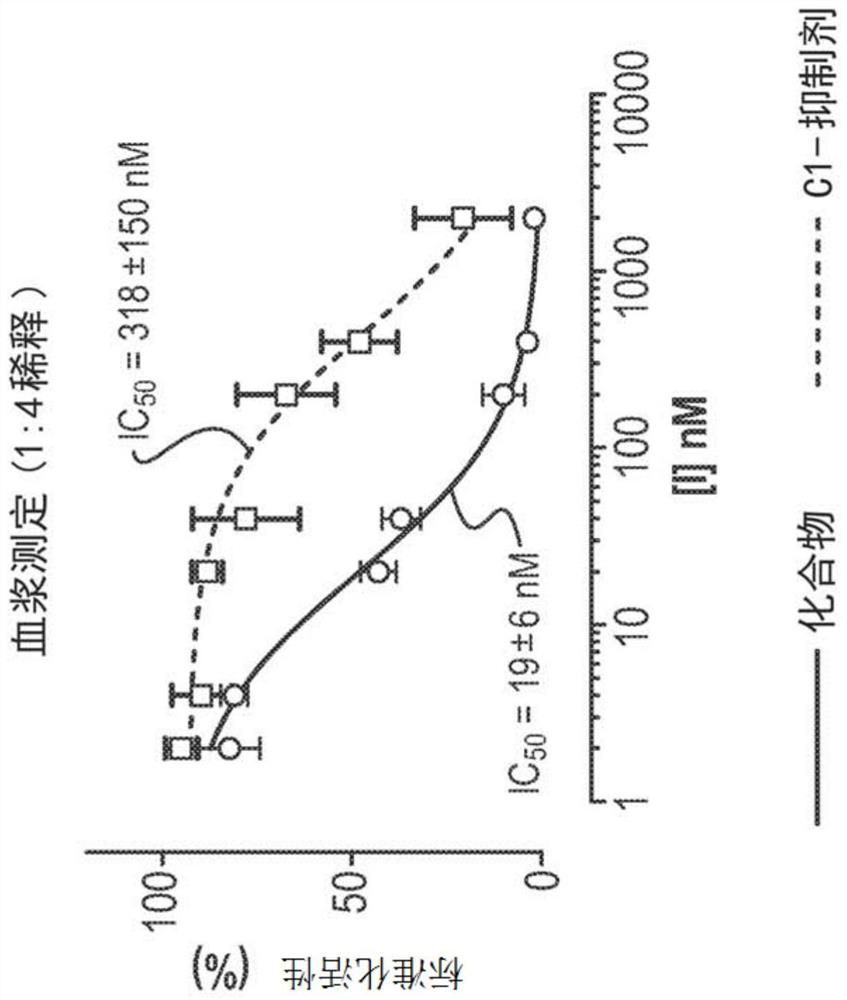
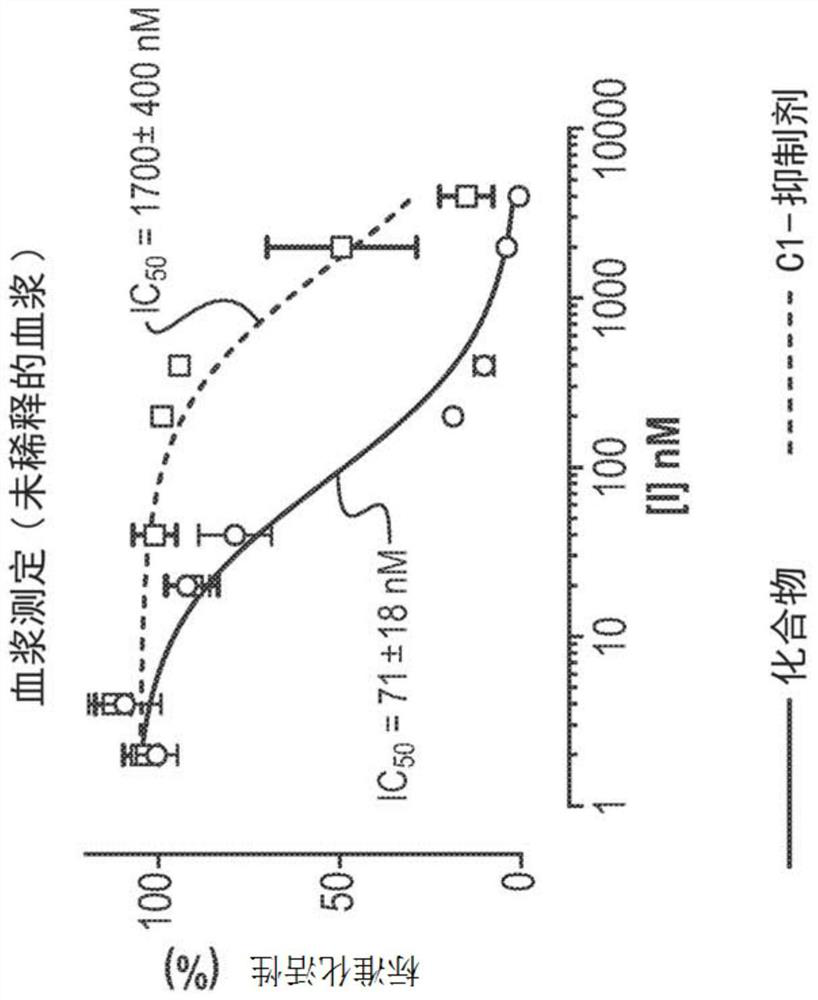
[0197] Example 3 - Comparison of Compounds of Formula A with C1 Inhibitors (C1-INH)
[0198] Purpose: To identify the biochemical and physiological properties of compounds of formula A that contribute to the control of optimal efficacy of the kallikrein kinin system in plasma. These properties were then compared to C1-INH (as a therapeutic benchmark for HAE).
[0199] method:
[0200] In vitro plasma kallikrein inhibitory activity was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8, 185; Shori et al., Biochem. Pharmacol., 1992, 43, 1209; Stürzebecher et al. , Biol. Chem. Hoppe-Seyler, 1992, 373, 1025). Human plasma kallikrein (Protogen) was incubated at 25°C with the fluorogenic substrate H-DPro-Phe-Arg-AFC and various concentrations of test compounds. Residual enzyme activity (initial reaction rate) was determined by measuring the change in absorbance at 410 nm and determining the IC of the test compound 50 value.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com