Evaluation, assays and treatment of PKAL-mediated disorders
一种介导、病症的技术,应用在PKAL-介导的病症的评估、测定和治疗领域,能够解决缓激肽过量产生等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
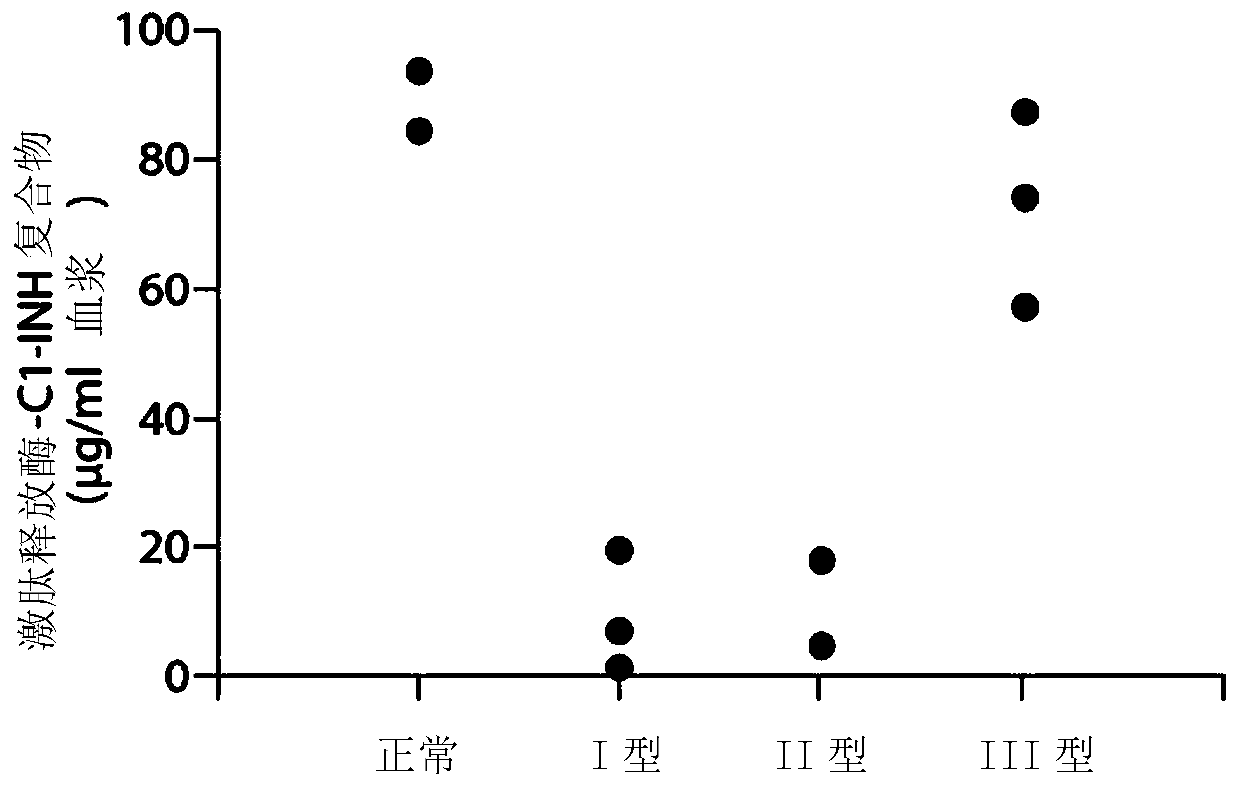
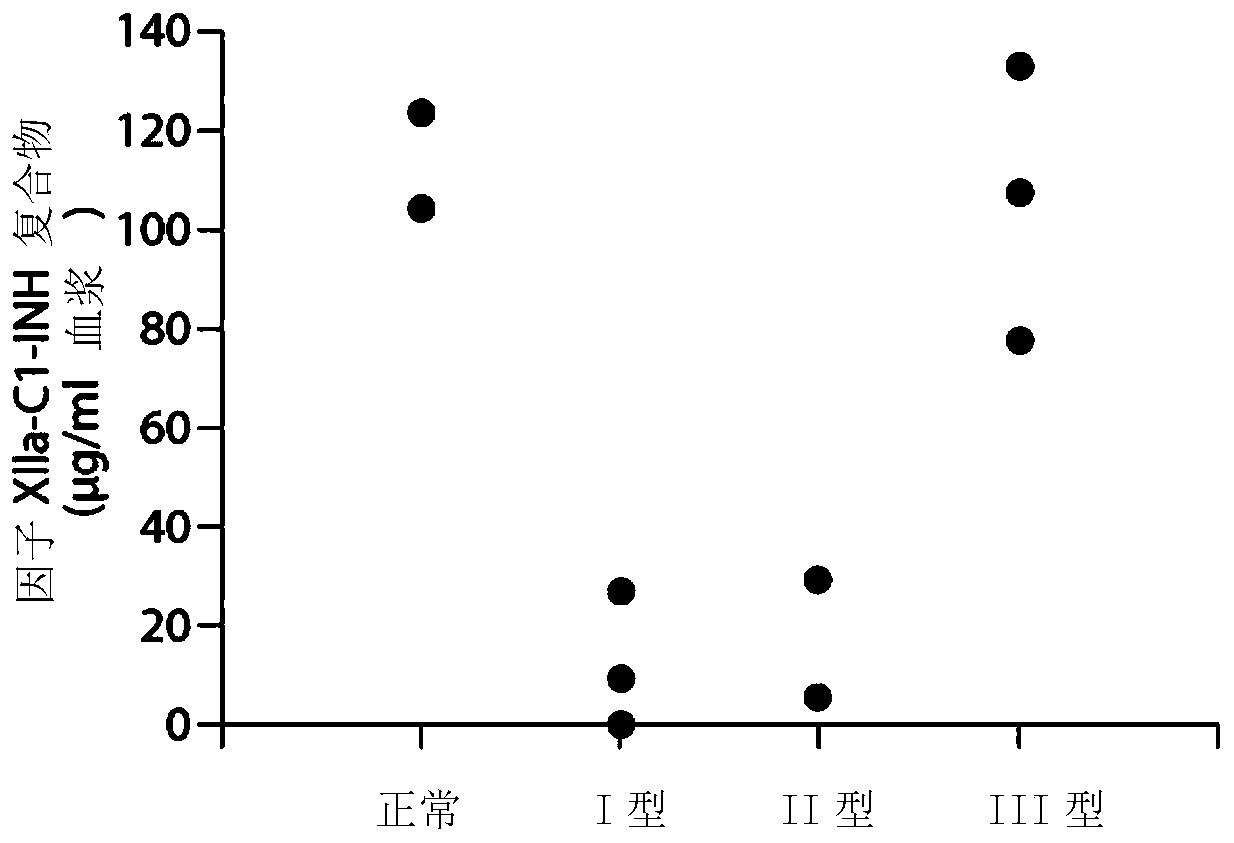
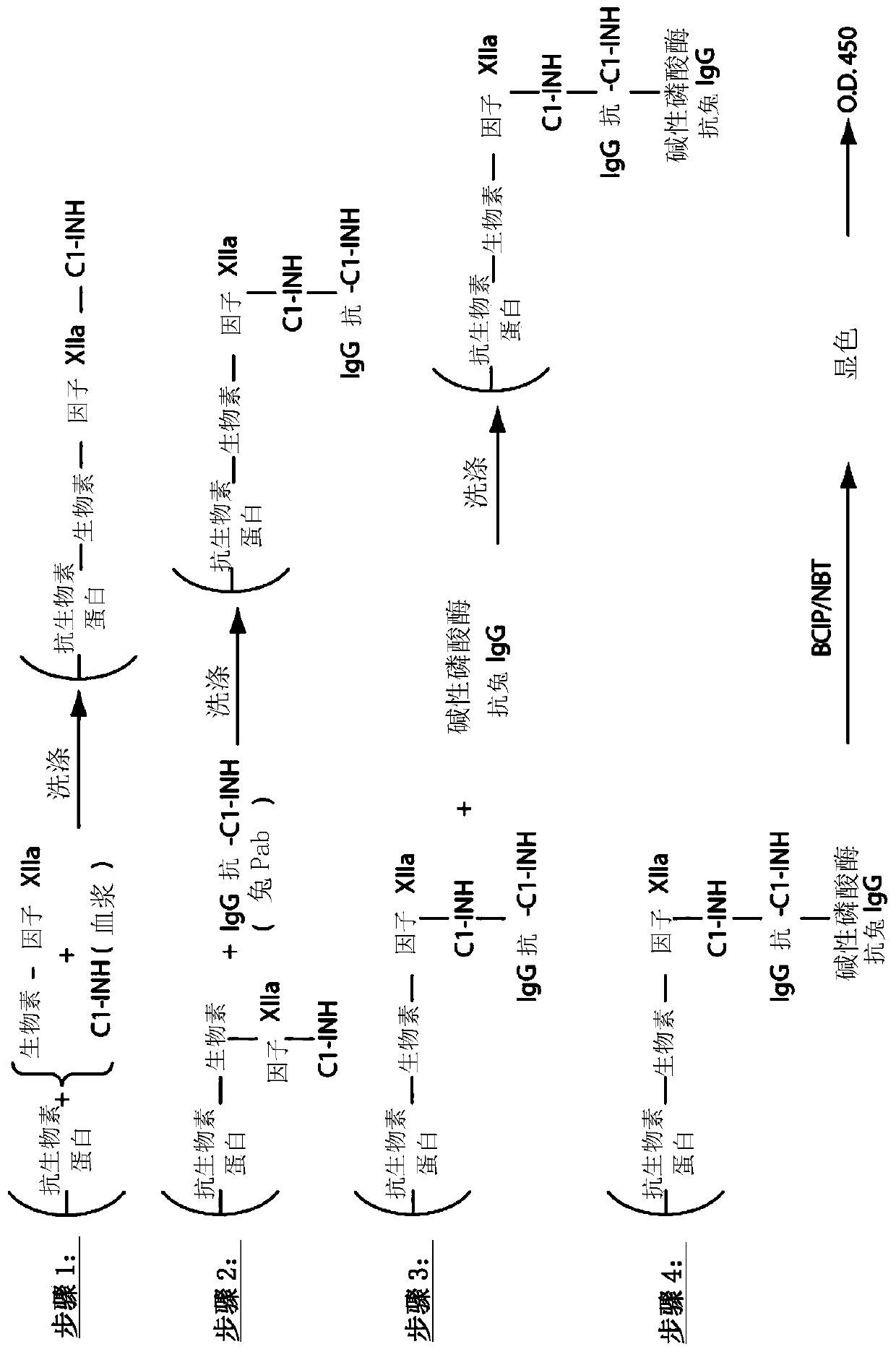
[0258] Example 1. Composite ELISA for Quantification of Functional Cl-INH in Plasma Using Activated Factor XII and / or Plasma Kallikrein
[0259] Dysfunction of the C1 inhibitor, C1-INH, has been demonstrated in hereditary angioedema type II (HAE), which renders the inhibitor ineffective. Type I HAE has low total C1-INH protein levels. Type III HAE is associated with normal C1-INH levels. (“Enzymatic pathways in the pathogenesis of hereditary angioedema: Therole of C1 inhibitor therapy.” Kaplan, A., The Journal of Allergy and Clinical Immunology 126(5):918-25 2010). C1-INH inhibits Factor XIIa, Fragment of Factor XII (XIIf), kallikrein and plasmin. In the absence of C1-INH function, marked activation of the bradykinin formation cascade leads to severe angioedema. Type I HAE is usually characterized by decreased total C1-INH levels. Type II HAE is usually characterized by normal to increased C1-INH levels, however the function of C1-INH is abnormal. The mechanisms leading t...
Embodiment 2
[0265] Example 2. Diagnostic Assay for Hereditary Angioedema Based on Inhibition of Activated Factor XII and / or Plasma Kallikrein
[0266] method
[0267] Patient and Sample Collection: Diagnosis of HAE is made by clinical presentation, low C1-INH protein and / or functional levels (using commercial assays). Citrated plasma from 42 patients with HAE and 23 healthy controls was separated by centrifugation of freshly collected blood at 2000 rpm for 10 minutes at 4°C. All samples were immediately aliquoted and stored at -80°C. Samples were handled similarly at all participating sites (Odense, Denmark; Budapest, Hungary) and shipped overnight on dry ice. The protocol was approved by the Ethics Committee (Ethics Committee) and the Data Protection Agency (Data Protection Agency) of the two participating sites.
[0268] Purified human Factor XIIa and kallikrein were obtained from Enzyme Research Laboratories (South Bend, IN), biotinylation reagents were obtained from Thermo Scientif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com