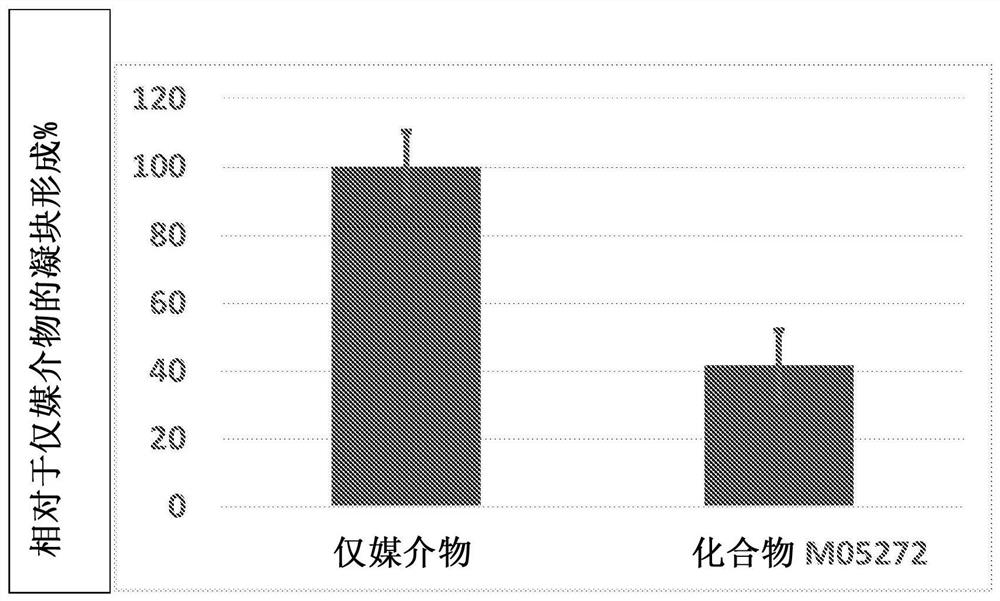
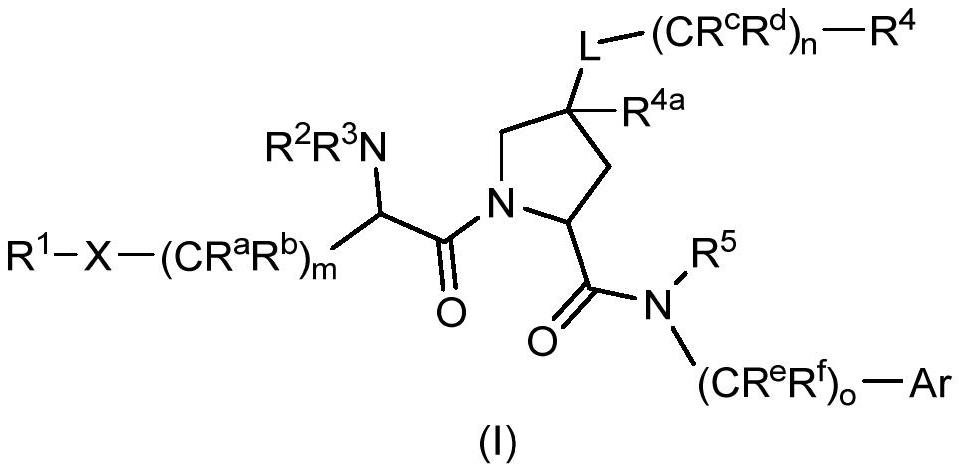
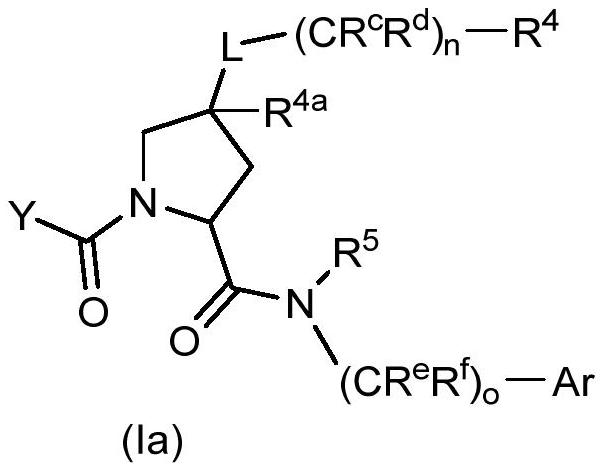
Factor xiia inhibitors
A kind of atomic, selected technology, applied in the field of producing the compound of the present invention, can solve the problem of not being suitable for long-term anticoagulation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0316] For some steps of the preparation methods of the compounds of the present invention it may be necessary to protect potentially reactive functions which are not reacted and thus cleavage of the protecting group. In this case, any compatible protecting group can be used. In particular, protection and deprotection methods such as those described by T.W. GREENE (Protective Groups in Organic Synthesis, A. Wiley-Interscience Publication, 1981) or P.J. Kocienski (Protecting groups, GeorgThieme Verlag, 1994) can be used. All of the above reactions used in the previous methods and the preparation of novel starting materials are conventional and references to literature precedents and examples and preparations relating thereto, appropriate reagents and reaction conditions for their performance or preparation and separation of desired The procedures for the production are well known to those skilled in the art.
[0317] Additionally, the compounds of the invention, as well as int...
Embodiment and
[0332] Example and Synthesis
[0333] 1H-NMR: Spectra were acquired on a Bruker DRX 400MHz or Jeol ECS 400MHz spectrometer. Spectra were measured at 294K (unless otherwise noted) and referenced to TMS (0.0 ppm), DMSO-d6 (2.50 ppm), CDCl3 (7.26 ppm), and chemical shifts are reported in parts per million (ppm) ( δ-value). Reports coupling constants (J) in hertz (Hz) and expresses spectral splitting patterns as singlet (s), doublet (d), triplet (t), quartet (q), multiplet, or more Overlap signal (m), broad signal (br); solvents are provided in parentheses.
[0334] abbreviation
[0335] The following abbreviations are used in the examples and other descriptive sections.
[0336] ABCN: azobicyclohexanenitrile; Boc: tert-butoxycarbonyl; Cbz: benzyloxycarbonyl; DavePhos: 2-dicyclohexylphosphine-2'-(N,N-dimethylamino)biphenyl; dba: three (dibenzylideneacetone); DBU: 1,8-diazabicyclo[5.4.0]undec-7-ene; DCE: 1,2-dichloroethane; DCM: dichloromethane; DIAD: Diisopropyl azodicarboxy...
Embodiment
[0659] Example Procedure X: Synthesis of (R)-4-(1-methyl-1H-pyrazol-4-yl)-tetrahydropyrrole-1,2-dicarboxylic acid 1-tert-butyl ester
[0660]
[0661] Step 1: Triflate of (R)-4-trifluoromethanesulfonyloxy-2,5-dihydro-pyrrole-1,2-dicarboxylate 1-tert-butyl 2-methyl ester form
[0662]
[0663] at -78°C, in N 2 Under atmosphere, lithium bis(trimethylsilyl)amide (1 M solution in THF, 44.0 mmol, 44.0 mL) was added dropwise to (R)-4-oxo-tetrahydropyrrole-1 over 20 minutes, In a stirred solution of 1-tert-butyl 2-dicarboxylate 2-methyl ester (40.0 mmol, 9.72 g) in anhydrous THF (50 mL). The resulting solution was stirred at -78°C for 1 hour, then a solution of N-phenyl-bis(trifluoromethanesulfonamide) (44.0 mmol, 15.7 g) in anhydrous THF (40 mL) was added dropwise over 20 minutes. The resulting solution was stirred at -78°C for 2 hours, then allowed to warm to room temperature over 2 hours. Saturated aqueous ammonium chloride (100 mL) was added to the reaction mixture, fol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com