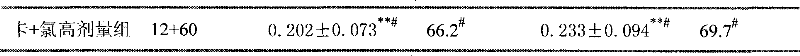Pharmaceutical composition containing clopidogrel and carbazochrome sodium sulfonate
A technology of clopidogrel and carbosulfonate, which is applied in drug combinations, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve problems such as increased risk of bleeding, rebound cardiovascular events, and reduced patient compliance. Achieve the effect of reducing the risk of bleeding, good synergistic effect, and inhibiting platelet aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
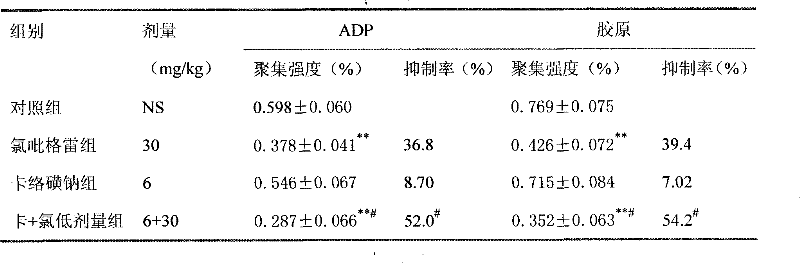
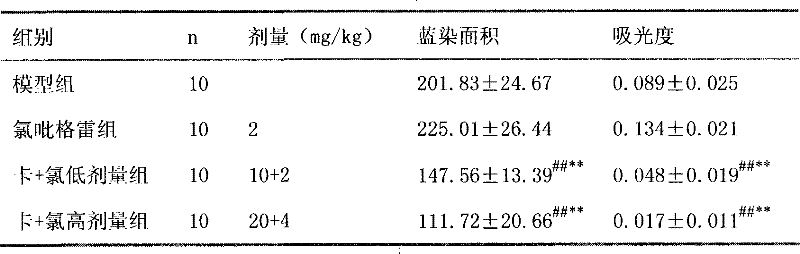
Image
Examples
Embodiment 1
[0012] Embodiment 1 common tablet
[0013] Sodium carbosulfonate 15g
[0014] Clopidogrel Bisulfate 75g
[0015] Microcrystalline Cellulose 250g
[0016] Lactose 20g
[0017] 10% starch slurry appropriate amount
[0018] Magnesium stearate 0.8g
[0019] Preparation process: Weigh the prescription amount of sodium carbosulfate, clopidogrel bisulfate, microcrystalline cellulose, and lactose and mix evenly. In addition, add an appropriate amount of 10% starch slurry to the mixed powder, mix evenly, make soft material, pass through a 18-mesh nylon sieve to make wet granules, and dry at about 60°C. The moisture content of the dry granules should be controlled below 1.5%. Sieve through a 20-mesh sieve, mix with magnesium stearate, and press into tablets.
Embodiment 2
[0020] Embodiment 2 common tablet
[0021] Sodium carbosulfate 75g
[0022] Clopidogrel Hydrochloride 15g
[0023] Starch 140g
[0024] Dextrin 20g
[0025] 50% ethanol appropriate amount
[0026] Magnesium Stearate 1.0g
[0027] Preparation process: Weigh the prescription amount of sodium carbosulfonate, clopidogrel hydrochloride, starch and dextrin and mix evenly. In addition, add an appropriate amount of 50% ethanol to the mixed powder, mix evenly, make soft material, pass through a 18-mesh nylon sieve to make wet granules, and dry at about 60°C. The moisture content of the dry granules should be controlled below 1.5%. Sieve through a 20-mesh sieve, mix with magnesium stearate, and press into tablets.
Embodiment 3
[0028] Embodiment 3 Dispersible tablets
[0029] Sodium carbosulfonate 50g
[0030] Clopidogrel 2.5g
[0031] Croscarmellose Sodium 10g
[0032] Microcrystalline Cellulose 150g
[0033] Polyvinylpyrrolidone 5.5g
[0034] 5% PVP 60% alcohol solution appropriate amount
[0035] Micronized silica gel 5g
[0036] Preparation process: Weigh carbosulfonium sodium and clopidogrel according to the prescription, use microcrystalline cellulose as filler, cross-linked carmellose sodium and polyvinylpyrrolidone as disintegrants, 5% PVP 60% alcohol solution It is used as binder, and micronized silica gel is used as flow aid. It is granulated in one step in a fluidized bed, and then compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com