Target spot for treating septicemia
A septicemia and target technology, applied in the field of septicemia treatment, can solve problems such as side effects and achieve the effect of improving living conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
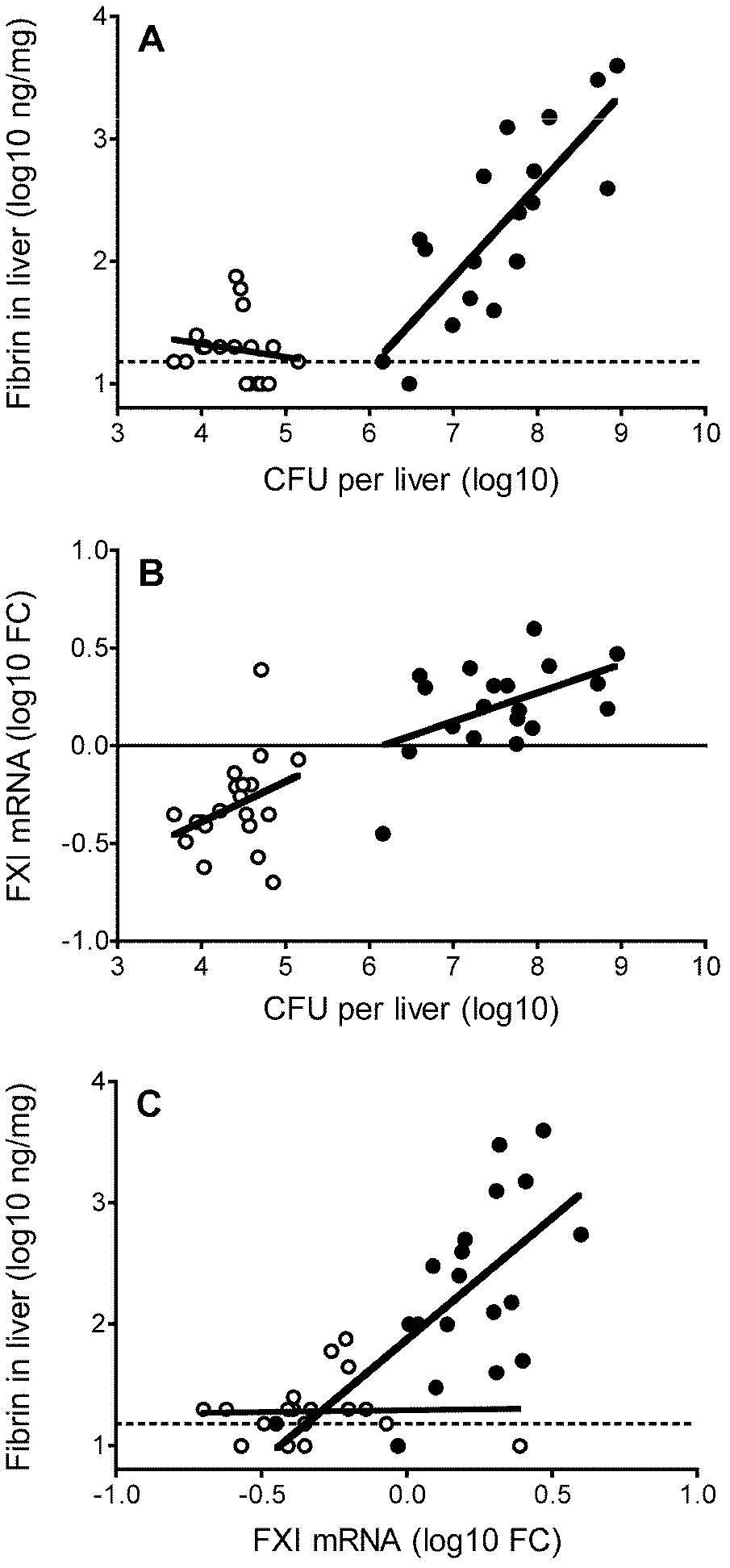
[0033] Example 1. Confirmation and verification of Factor XI as a therapeutic target for sepsis
[0034] 1. Detection of mRNA gene chip after low-dose (sublethal) and high-dose (lethal) Listeria infection:
[0035] (1) Intraperitoneally injected Listeria monocytogenes (L.monocytogenes, EGD strain) into C57BL / 6 mice, passaged, isolated spleen, ground TSA and cultured, picked monoclonal TSB, cultured, counted, and stored in -70°C refrigerator for later use. The LD50 of this strain is 2×10 6 CFU. Dilute cryopreserved bacteria in PBS to the desired infectious dose, with a low dose of 0.6-1.0 x 10 5 CFU, high dose 2.5-2.7×10 6 CFU, the final injection volume was 200 μl.
[0036] (2) C57BL / 6 mice were raised in SPF (specific pathogen free) laboratory for 6-8 weeks, and the food, water and excipients were changed regularly. The 15 mice were divided into three groups and injected with 200 μl low and high doses of Listeria, respectively, and a 200 μl PBS blank control group was es...
Embodiment 2
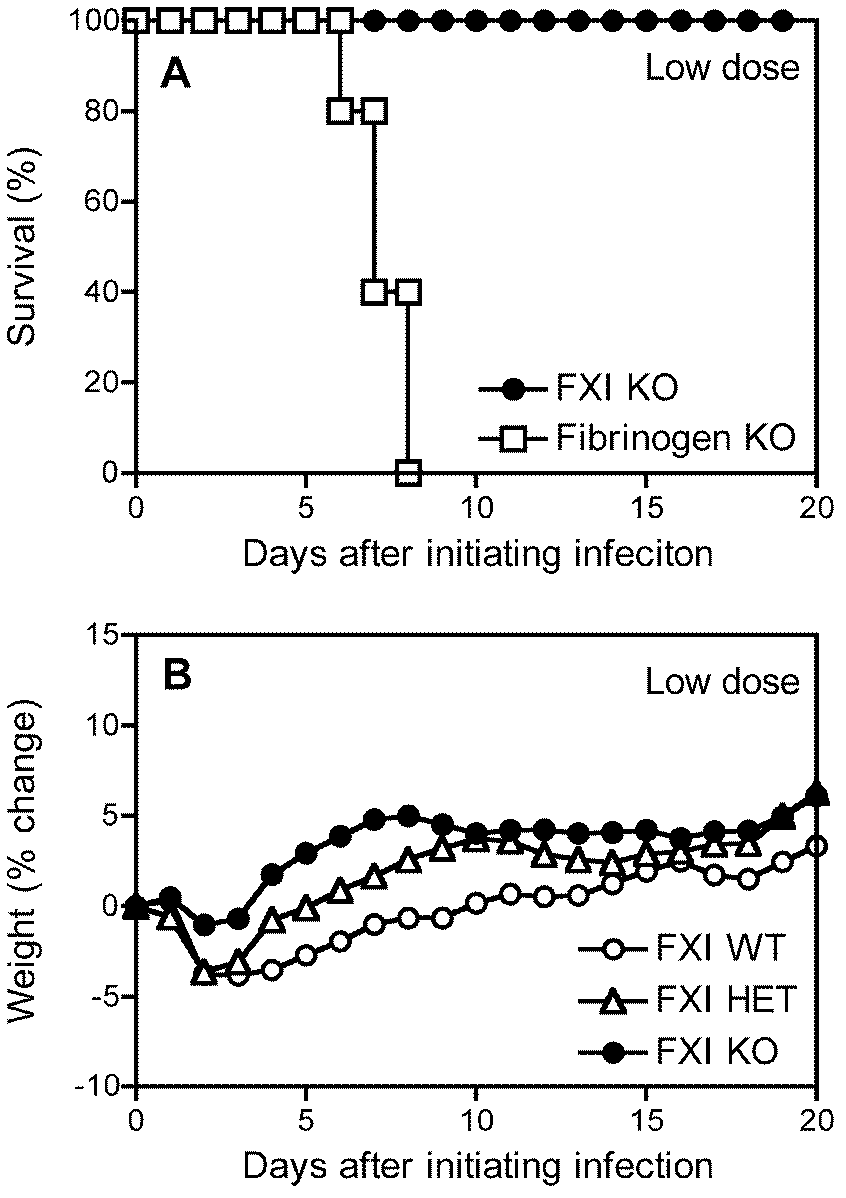
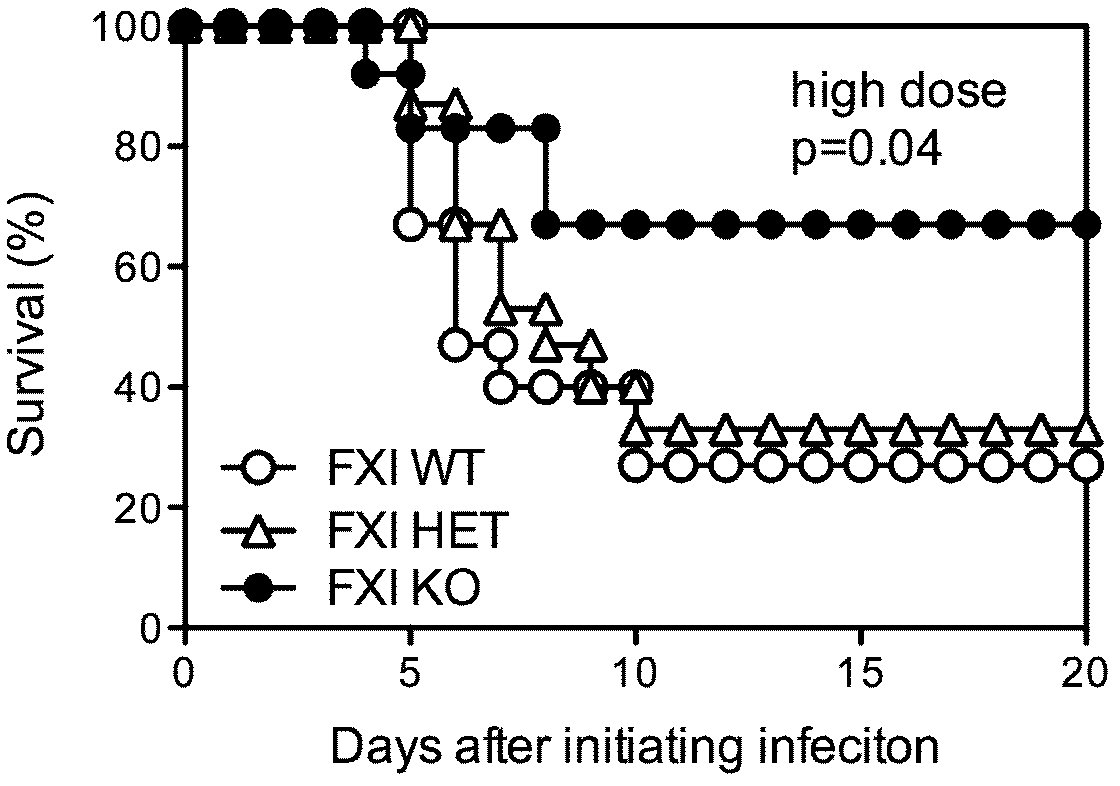
[0057] Example 2. Discussion on the mechanism of Factor XI as a therapeutic target for sepsis
[0058] (1) Intraperitoneally injected Listeria monocytogenes (L.monocytogenes, EGD strain) into C57BL / 6 mice, passaged, isolated spleen, ground TSA and cultured, picked monoclonal TSB, cultured, counted, and stored in -70°C refrigerator for later use. The LD50 of this strain is 2×10 6 CFU. Use PBS to dilute the cryopreserved bacteria to the required infectious dose, the final injection volume is 200 μl, the so-called low dose in this example is 0.6-1.0 × 10 5 CFU, high dose is 2.5-2.7×10 6 CFU;
[0059] (2) Factor XI HET mice were mated and screened to 6-8 weeks. There were 5 strict control mice in each group, namely Factor XI WT, Factor XIHET, and Factor XI KO. They were raised in an SPF (specific pathogen free) laboratory, and their food was changed regularly. , water and excipients. 200 μl of low-dose and high-dose Listeria were injected respectively, and a 200 μl PBS blank ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com