Preparation method and application of specific antibody containing milk or serum produced by novel coronavirus immunized cows
A coronavirus and immune preparation technology, applied in the direction of antiviral immunoglobulin, antibody, antiviral agent, etc., can solve the problems of not being popularized, not suitable for blood donation, restrictions, etc., to reduce mortality, prevent virus from invading cells, The effect of reducing virus content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of milk and serum containing novel coronavirus antibodies:
[0028] The sequence of the novel coronavirus hCov-19 / Wuhan / ZY38-1 / 2020 strain was submitted to the GISAID database on July 10, 2020, and the accession number is EPI_ISL_486645. In the present invention, the virus is referred to as novel coronavirus ZY38-1 for short.
[0029] 1. Proliferation, culture and inactivation of novel coronavirus ZY38-1
[0030] (1) Cell culture:
[0031] VERO cells were cultured in DMEM containing 10% FBS for about 48 hours;
[0032] Cell subculture: digest with 0.25% trypsin during subculture, and the cells can be digested in about 10 minutes.
[0033] Specific steps are as follows:
[0034] For adherent cells, use a dropper or pipette to suck off the old culture medium in the culture vessel, and wash away the residual old culture medium with serum-free DMEM medium;
[0035] Add 1-2ml of digestive solution (0.25% trypsin) to the bottle and gently shake the culture bot...
Embodiment 2
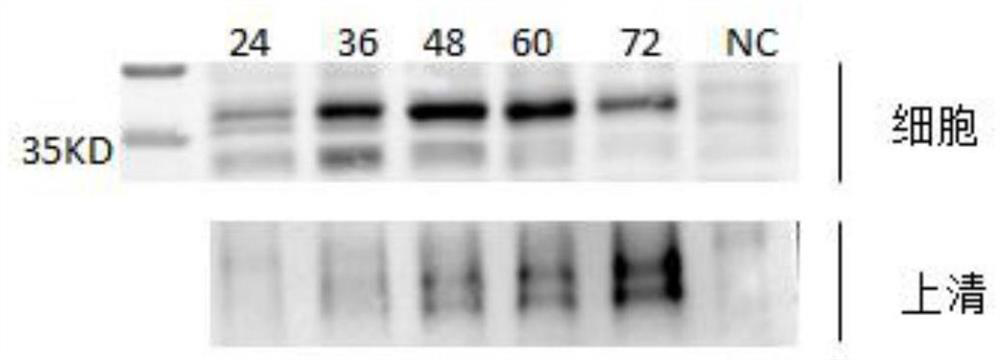
[0056] Determination of Specific Antibody Levels in Immune Milk and Immune Serum
[0057] 1. Expression and purification of novel coronavirus RBD antigen protein
[0058] Through the design of expression elements and vector reconstruction, the RBD gene of the new coronavirus was inserted into the pCMV3 expression vector to construct the pCMV3-RBD-his eukaryotic expression vector, and the target protein was obtained by transient expression in 293T suspension cells. 1.1 Construction of pCMV3-RBD-his plasmid
[0059] Extract inactivated ZY38-1 virus RNA, reverse transcription and amplify viral cDNA, use cDNA template for RBD fragment amplification, amplification primer RBD-F: 5'-ATGAGGGTCCAACCAACAGAGAGC-3'; RBD-R: 5'-GAAGTTCACACACTTGTTCTTCAC -3'. In order to facilitate subsequent RBD protein purification, 6×HIS sequence was added to the back end of RBD gene to obtain RBD-his gene. The pCMV3 empty vector and RBD-his gene were double digested with KpnI and XbaI, and the fragment...
Embodiment 3
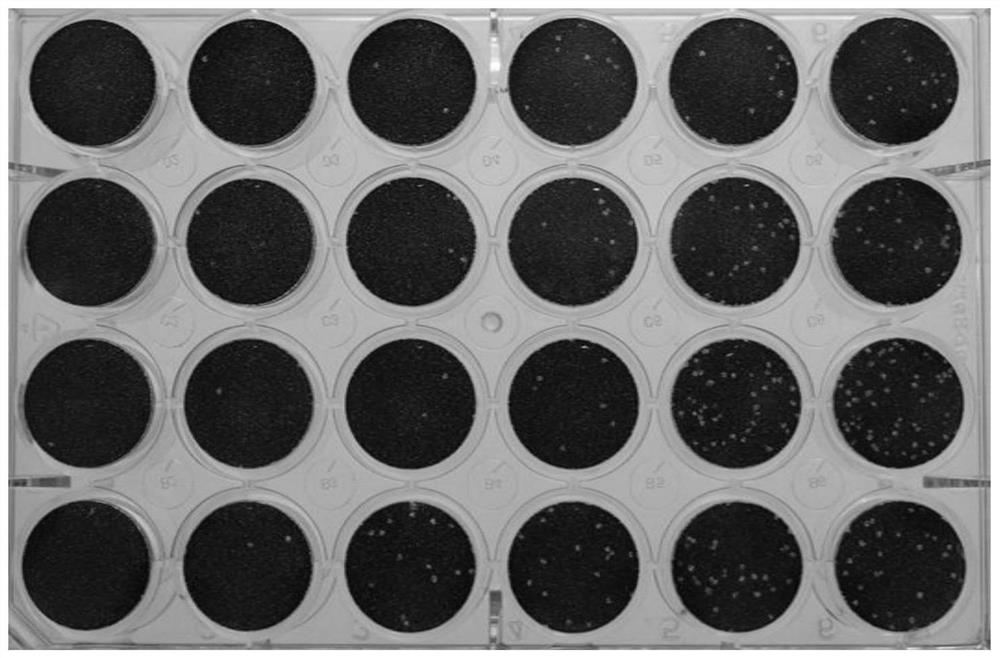
[0083] Neutralizing antibody titer of immune milk and immune serum
[0084] The new coronavirus plaque reduction micro-neutralization test was used to detect the neutralizing antibody titer of the antibody in the immune milk and immune serum to the new coronavirus. The specific steps are as follows:
[0085] (1) Passage Vero cells in good growth state to 12-well cell culture plate, 3-5E5 / well, and grow into a dense monolayer the next day.
[0086] (2) Inactivate the serum and milk samples processed in Example 2 and stored at -20°C for 30 minutes at 56°C.
[0087] (3) Serum and milk samples were diluted with serum-free DMEM medium in a 24-well plate, and the number of 24-well plates was prepared according to the number of samples, with 4 samples per plate, and a virus back-titration control was added.
[0088] (4) Add 441ul or 405ul DMEM to the first column of each well, and add 300ul to other wells.
[0089] (5) Add 9 ul of serum or 45 ul of milk sample to the wells in the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com