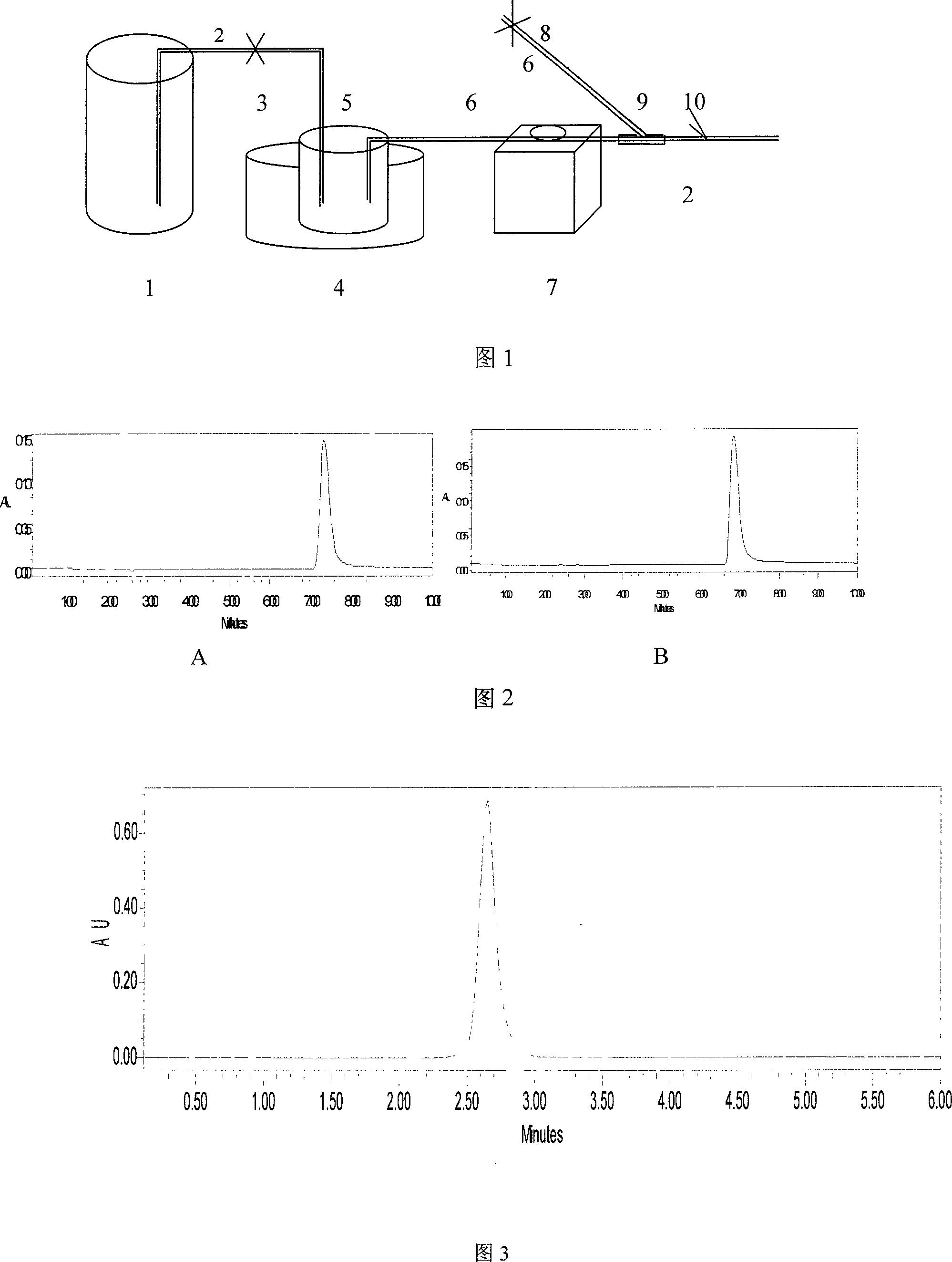Sodium citrate injection for tube-enveloping and method for preparing the same
A technology of sodium citrate and injection, which is applied in the fields of beverage, food, and medicine. See High Concentration Sodium Citrate Products and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0332] 1.4.1 Preparation of Plasma Take 9ml of fresh blood from the heart of New Zealand white rabbits, use 1ml of 3.28% sodium citrate solution for anticoagulation, mix and centrifuge (10min, 700r / min, 37℃), the supernatant is rich in platelets Plasma (PRP). The remaining liquid was centrifuged again (10min, 2000r / min, 37°C), and the supernatant was platelet-poor plasma (PPP).
[0333] 1.4.2 Preparation of Heparin Sodium Solution Take 1ml of heparin sodium injection and mix it with normal saline according to the half-dilution method to make 50mg / ml~1.5625mg / ml (125 units are equivalent to 1mg, that is, the specification of heparin sodium injection is 2ml:100mg ) 6 concentrations of heparin sodium solution.
[0334] 1.4.3 Determination of the maximum platelet aggregation rate PAG(M) With 200μl PRP plus 10μl different test drugs, pre-warm at 37°C for 5min, add 6μl ADP to induce, and measure the platelet aggregation rate.
[0335] 1.4.4 Determination of APTT, PT and TT, respectively...
Embodiment 1
[0467] Example 1. Preparation of Sodium Citrate Injection for Tube Sealing
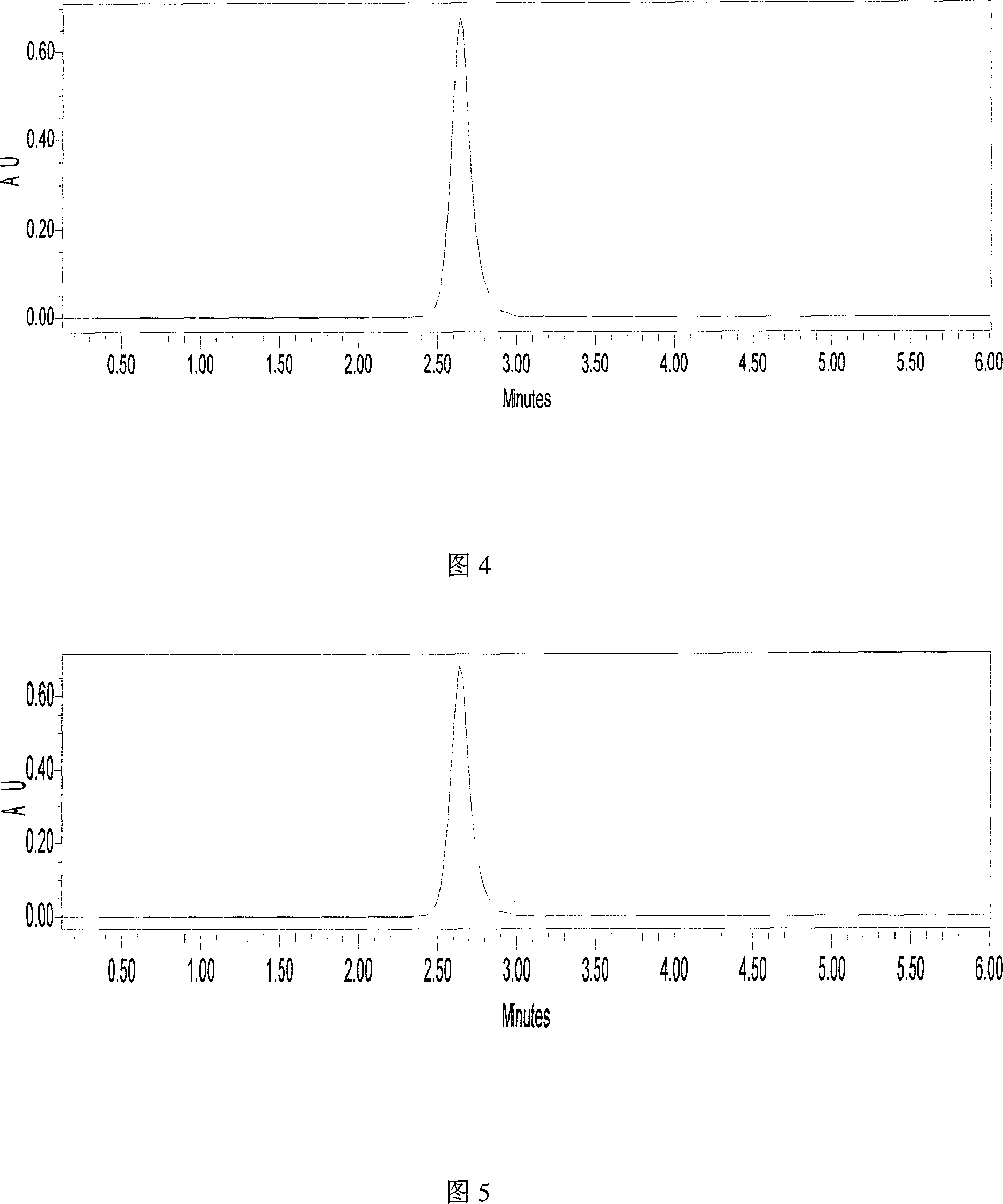
[0468] According to the prescription, weigh the raw materials according to the weighing SOP; take an appropriate amount of hot water for injection, add sodium citrate and citric acid and stir to dissolve, add the water for injection to the full amount, stir well; plate and frame filter for decarbonization filtration, the filter material is Microporous filter membrane, pore size 1.2μm, 0.8μm; microporous filter disc for terminal filtration, filter material is microporous filter membrane, pore size 0.65μm; intermediate quality control content: content is 97.0%-103.0% of the labeled amount, pH value 6.8-7.5; Filling; check and seal; temperature 110℃, time 20 minutes, autoclave sterilization; put the ampoule before the clarity inspection of 2000-3000LX illumination, inspect it visually, perform according to the requirements of clarity inspection, light inspection The visual acuity of the personnel should be a...
Embodiment 2
[0469] Example 2. Clinical application of the sodium citrate injection for tube sealing 1
[0470]To study the effect of 47% sodium citrate solution on long-term indwelling catheter sealing in hemodialysis patients. Forty patients with uremic maintenance dialysis with long-term indwelling catheters were selected and randomly divided into citric acid test group with 20 cases and heparin control group with 20 cases. After each dialysis, the test group was sealed with 47% sodium citrate solution, and the control group was sealed with heparin sodium solution. Both of them were used continuously for 6 months. The patency of the catheter was recorded 6 months before and 6 months before the medication, and the catheter-related infections were observed. Happening. Before the medication and at the end of January, March, and June, the arterial and venous end-sealing fluids were drawn for bacterial culture. The electrolytes were measured before the patient's medication, and 2 weeks and 6 mon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com