Multi-primer and kit for rapidly detecting influenza A, influenza B and novel coronavirus
A technology of influenza B virus and influenza A virus, which is applied in the field of multiple primers and kits for rapid detection of influenza A, influenza B and new coronavirus, can solve the problems of high laboratory environment requirements and long time consumption , to achieve the effects of improving detection efficiency, avoiding cross-infection, and high reference value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Design and optimization of specific primer probes.
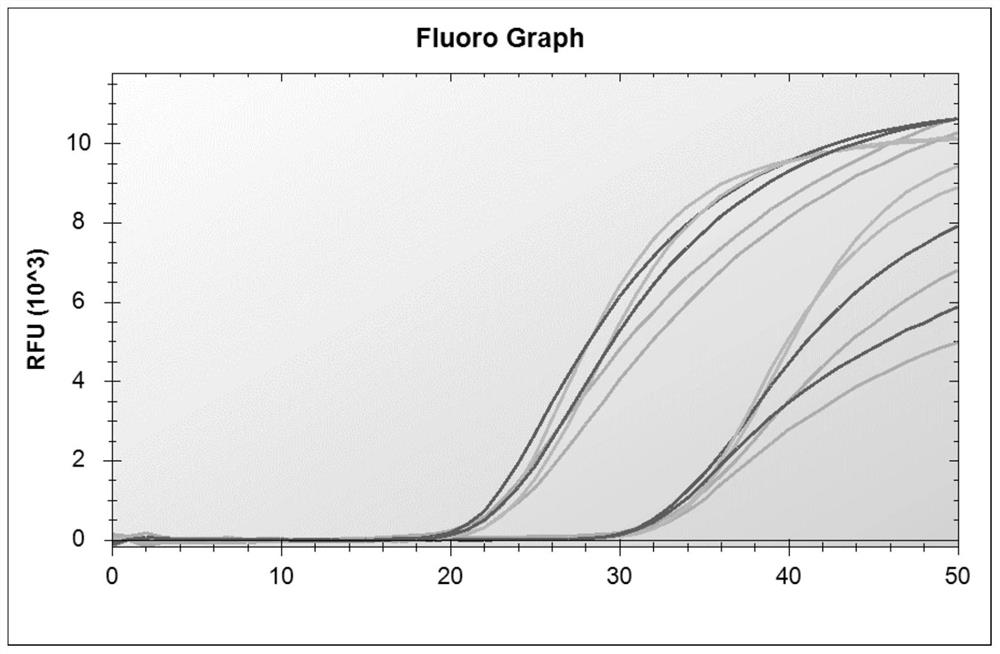
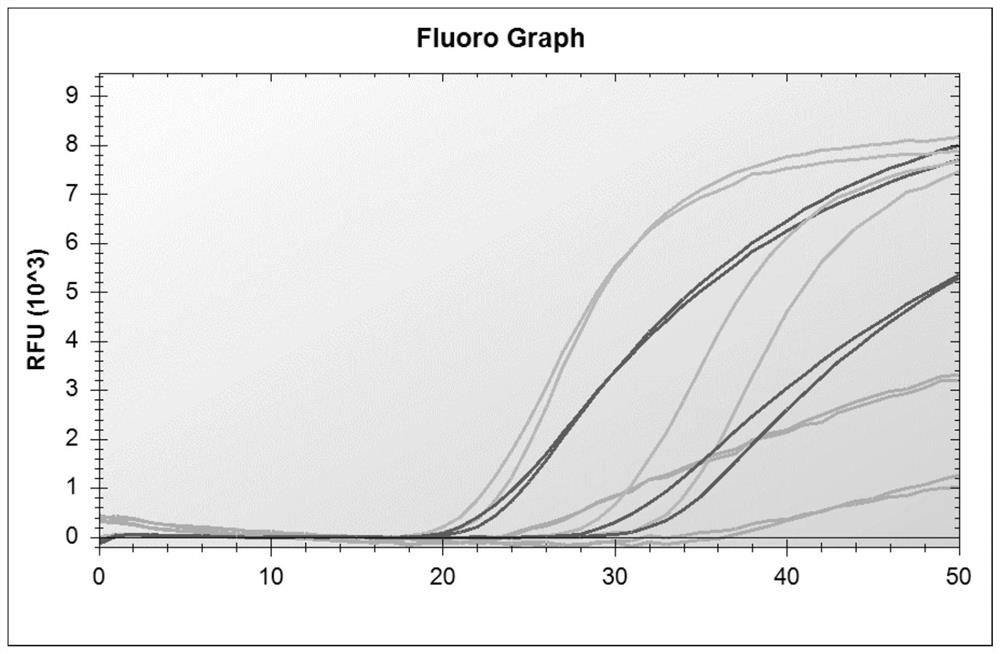
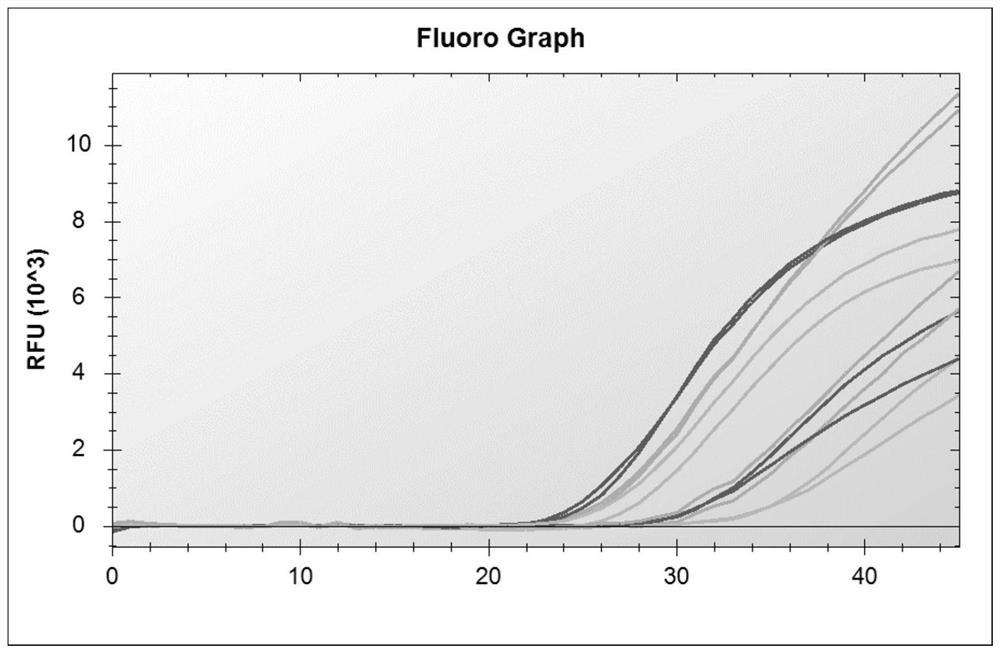
[0060] Referring to the influenza diagnosis and treatment guidelines and the new coronavirus diagnosis and treatment guidelines, nucleic acid testing is the gold standard for judging the diagnosis of new coronavirus infection. In order to ensure the detection of influenza A virus, influenza B virus and new coronavirus, according to the nucleic acid sequences of these three pathogens , analyzed the conserved regions of their genes, and finally selected primer probes for the conserved regions of the M gene of influenza A virus, the NS gene of influenza B virus, and the N gene of the new coronavirus, in order to obtain multiplex genes with better specificity and sensitivity. Primer-probe system combination, designed a total of 60 primers and 28 probes, and combined multiple experiments. At the same time, human-derived internal reference genes were designed to monitor the specimen collection, nucleic acid extraction proce...
Embodiment 2
[0079] A kit for multiple rapid detection of influenza A virus, influenza B virus and novel coronavirus, comprising:
[0080] Reagent Ⅰ (735μl): Taq polymerase (0.04U / uL), MgCl 2 (4.5mM), dNTPs (0.2mM), and the primer concentrations of influenza A virus, influenza B virus and novel coronavirus are all 0.4μM, and the probe concentration is 0.25μM.
[0081] Reagent II (1500U): fast reverse transcriptase in freeze-dried form.
[0082] Negative quality control: normal saline.
[0083] Positive quality control product: pseudovirus containing influenza A virus, influenza B virus, new coronavirus and internal reference gene target fragment, the concentration of pseudovirus is 1.0×10 2 ~1.0×10 4 copies / μL.
Embodiment 3
[0085] The detection method of the kit for multiple rapid detection of influenza A virus, influenza B virus and novel coronavirus.
[0086] 1. Specimen type: oropharyngeal swab, nasopharyngeal swab.
[0087] 2. Nucleic acid extraction:
[0088] Use commercial RNA extraction kits, such as nucleic acid extraction reagents based on silica gel membrane spin column method or nucleic acid extraction reagents based on magnetic bead method, and operate according to the kit instructions, and finally collect 80 μl RNA solution for direct detection or storage in -80°C. Both negative quality control and positive quality control need to be extracted.
[0089] 3. Amplification reaction:
[0090] Each tube of a complete detection system should include 14.7 μL reagent I, 0.3 μL reagent II (in freeze-dried form, add 20 μL depc water to dissolve before use, and place it on ice for more than 10 minutes before use), 5 μL quality control material or nucleic acid of the sample to be tested . S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com