Preparation method of anti-influenza A H1N1 virus specific IgY and related formulation thereof
An influenza virus, avian influenza virus technology, applied in the preparation method of peptide, antiviral agent, antiviral immunoglobulin, etc., can solve the problem of unstoppable H1N1 influenza virus, central nervous system side effects, failure to detect, etc. problems, to achieve the effect of good immune conditioning and inhibition, and elimination of viral infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] 7. Preparation of Crude Extract
[0062] Firstly, according to the different birds to be immunized and the antigens used for immunization, the immunized eggs are classified and coded. Wash the immune eggs with running water, scrub and disinfect with alcohol, break the immune eggs with an egg beater, filter the egg whites with a yolk sieve, leave the egg yolks, and stir evenly; add distilled water to dilute and mix according to 4-6 times the volume of the egg yolk liquid Evenly, adjust the pH to 5.5-6.0 with 1.0N HCI solution.
[0063] The diluted solution with adjusted pH value is further fully stirred evenly, then cooled to 2-6°C, and left to stand for 12 hours to 24 hours; the diluted solution is centrifuged at 10,000rpm for 20 minutes; the supernatant obtained from separation is ultrafiltered Concentrate by ultrafiltration in a container for 10-20 times; then add sodium alginate solution, stir until cloudy; then add 1.0% CaCl 2 solution, stir evenly, let stand at 4...
experiment example 1
[0083] Experimental example 1 : Detection of specific IgY of anti-mutant influenza A (H1N1) virus and specific IgY of anti-influenza secondary infection bacteria
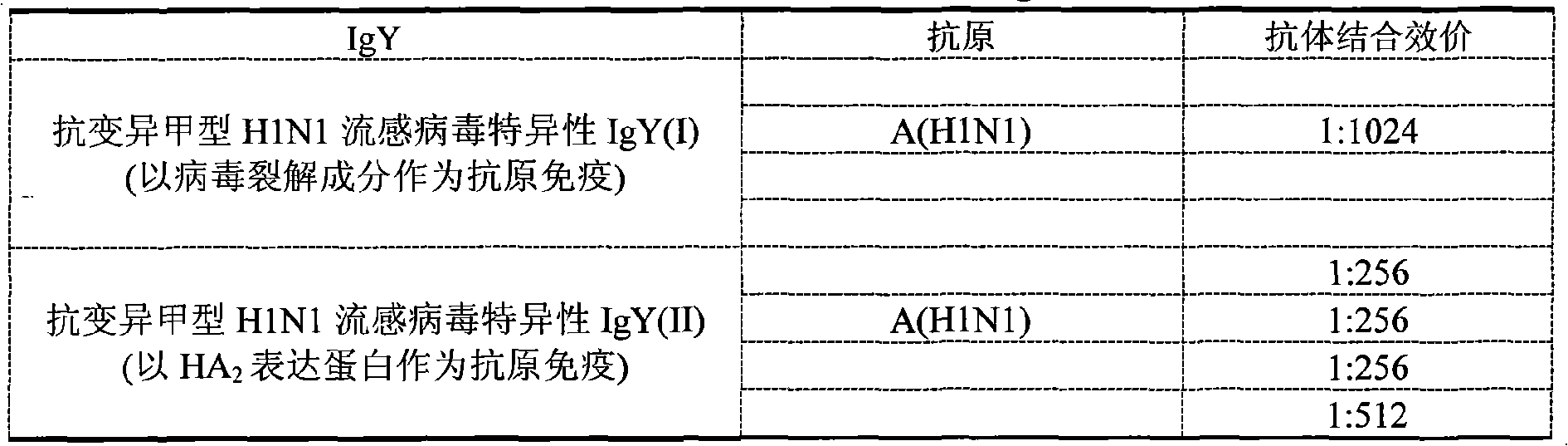
[0084]Using SDS-PAGE (sodium dodecyl sulfate-polypropylene gel) electrophoresis assay, the specific IgY of different anti-variant influenza A H1N1 influenza virus and the specificity of anti-influenza secondary infection bacteria prepared according to the above process respectively The crude IgY extract was detected, and the result contained 45-52% IgY. The crude IgY extract was passed through the column twice to obtain pure IgY. After SDS-PAGE analysis, the purity reached PAGE pure, as shown in the table below:
[0085] Pure IgY
experiment example 2
[0086] Experimental example 2 : Activity detection of specific IgY against mutated influenza A (H1N1) virus and specific IgY against bacteria secondary to influenza infection
[0087] The anti-variant H1N1 influenza virus-specific IgY and the anti-influenza secondary infection bacteria-specific IgY prepared by the technology invented by the inventors were tested by "ELISA" (enzyme-linked immunosorbent assay).
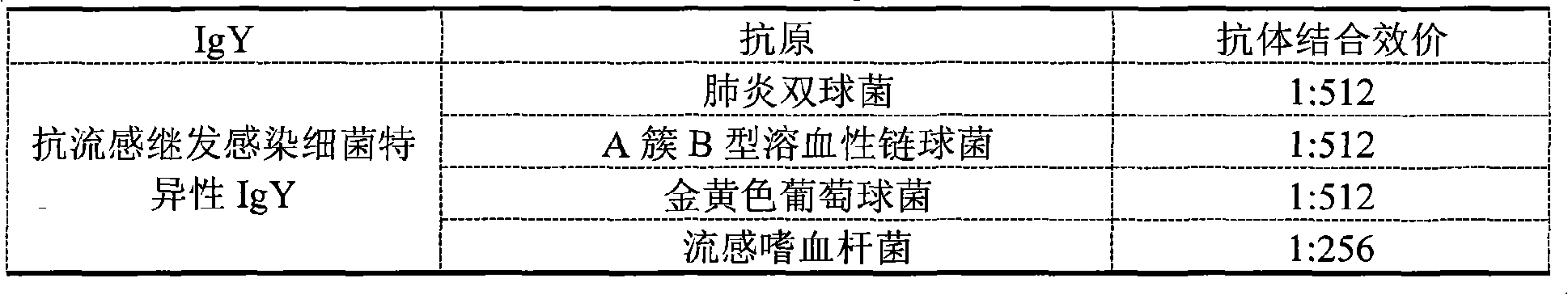
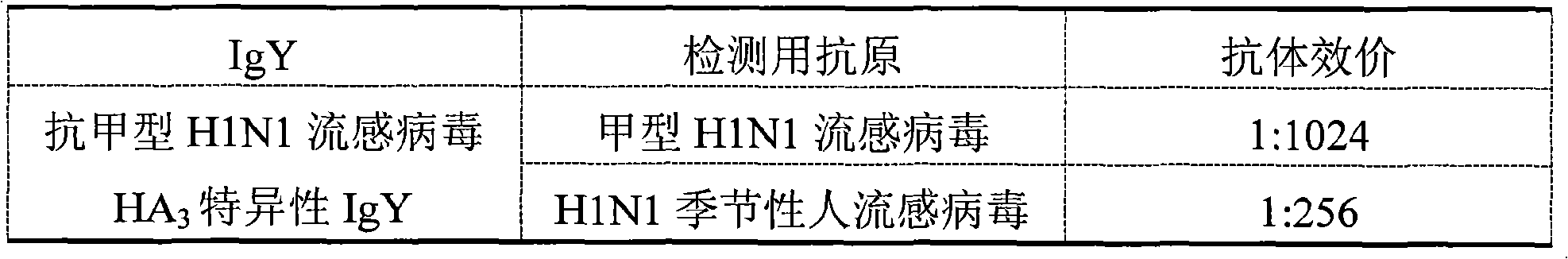
[0088] The test results show that, among the two different anti-variant H1N1 influenza virus-specific IgYs prepared, the antibody binding titer of type I to the influenza A H1N1 influenza virus has reached above 1:1024. And the antibody binding titer of type II to influenza A H1N1 virus also reaches above 1:512. Similarly, the anti-influenza secondary infection bacterial specific IgY antibody titers to representative pneumococci, group A group B hemolytic streptococcus, Staphylococcus aureus, and Haemophilus influenzae were all above 1:256.
[0089] Detection results...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com