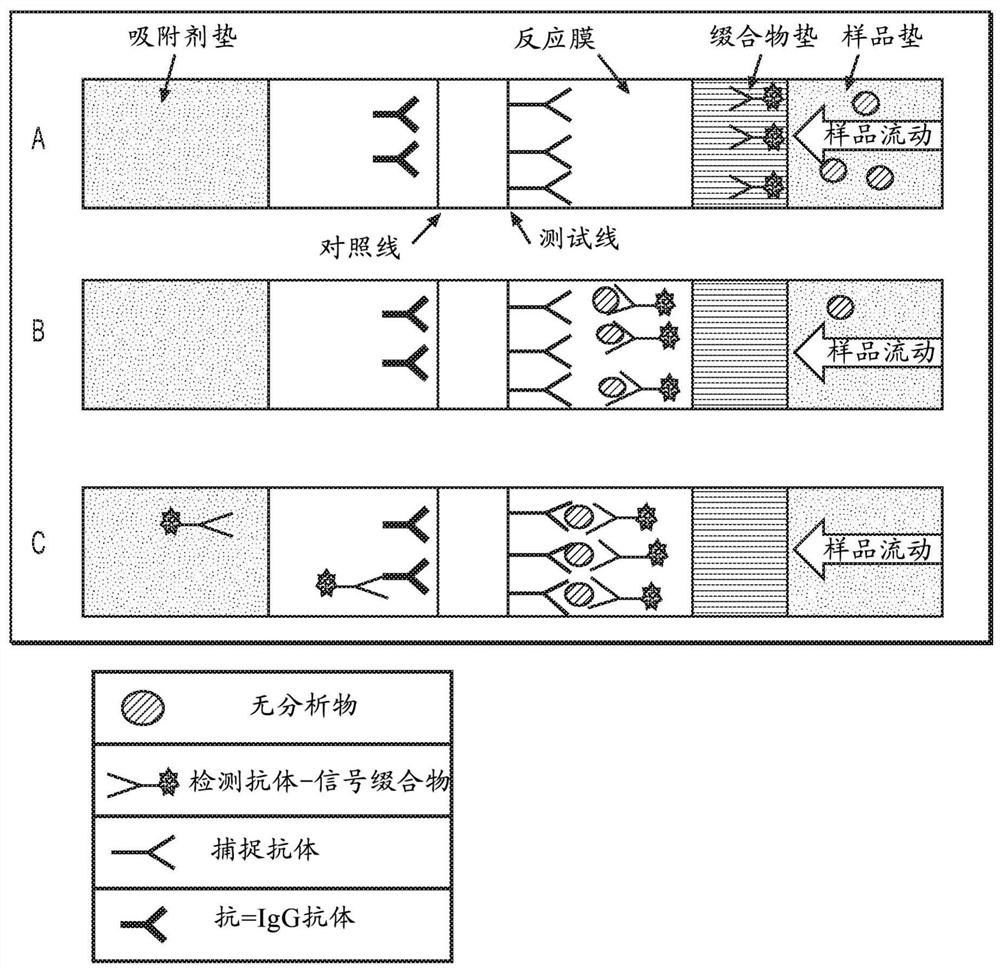
Antibody pairs for use in a rapid influenza a diagnostic test
A type of influenza A and antibody pair technology, applied in disease diagnosis, measurement devices, peptide sources, etc., can solve problems such as unavailable combination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] Example 1: Detection of Antibody Conjugate Dose Response to NP Antigens of H1N1 and H3N2 on Half (ie "Wet") Dip Tablets.
[0126] Capture antibody was striped onto 2.5 x 30 cm nitrocellulose membrane cards at a rate of 0.1 µl / sec using the Isoflow reagent dispensing module. The cards were dried overnight at ambient temperature in a humidity controlled drying room and then laminated with a 21 mm wide cellulose fiber core (222 Ahlstrom film). The cards were cut into 5 mm wide strips using a motion cutter (Kinematic Matrix 2360), resulting in 5 mm wide semi-lateral flow impregnated sheets ("semi-dipped sheets").
[0127] 20 μl each of the NP stock solution and the detection antibody-gold conjugate suspension were added to the 96-well plate. Place the half-dipped sample in the well and let stand until all the liquid is absorbed.
[0128] exist Figure 5 and 6 Shown above are the results of visual scoring of sample test line color intensity relative to a Rann scale of 0 ...
Embodiment 2
[0130] Example 2: Evaluation of antibody pairs (A) in "dry-up" format.
[0131] Nitrocellulose membranes were stripped with 1.0 mg / ml capture antibody and dried overnight at ambient temperature in a humidity-controlled environment. Spray the conjugate pad on the membrane card with detection antibody conjugate.
[0132] Using standard techniques, run the apparatus for 20 min with 60 μl of each H1N1 NP standard and detection antibody conjugate suspension.
[0133] Figure 7 The results of visually scoring the test lines relative to the Rann scale as previously described are shown in .
[0134] Antibody pair (A) has been shown to detect the NP antigen of the H1N1 strain to 1 ng / ml.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com