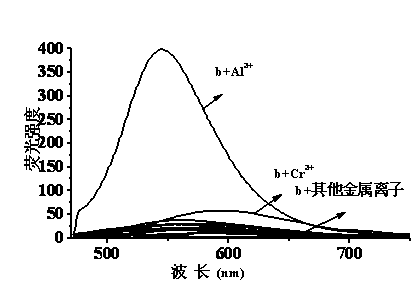
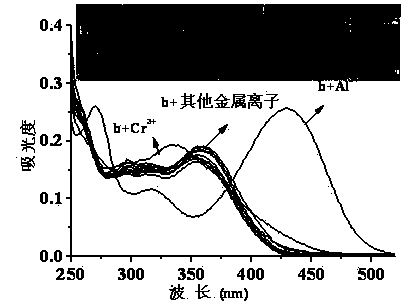
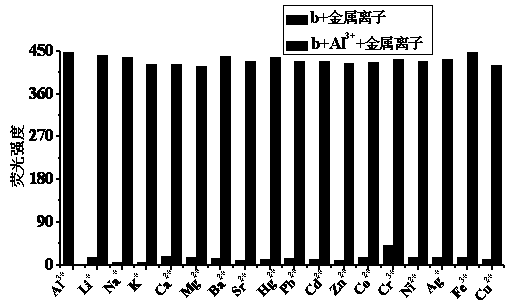
Quinaldine derivative b fluorescent and colorimetric reagent as well as preparation method and application thereof
A derivative, quinaldine technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., to achieve high yield, low detection limit, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Synthesis of intermediate a, namely (E)-2-(2,4-diacetoxyphenyl)vinyl-8-acetoxyquinoline:
[0066] In a 100ml three-necked flask under nitrogen protection, add 1.00g (6.29mmol) of 8-hydroxyquinaldine, 20ml of acetic anhydride and 1.74g (12.58mmol) of 2,4-dihydroxybenzaldehyde, heat to reflux for 5h, and concentrate to remove the solvent Acetic anhydride, purified by column chromatography, eluent: chloroform / ethyl acetate ( V:V =3:1) to obtain 1.78g of white target product a with a yield of 70.0%. m.p.131~133℃; 1 H NMR (400MHz, CDCl 3 )δ: 2.31(s, 3H, -COCH 3 ), 2.40(s, 3H, -COCH 3 ), 2.53(s, 3H, -COCH 3 ), 6.99(d, J=2.4Hz, 1H, ArH), 7.06-7.09(m, 1H, ArH), 7.30(s, 1H, ArH), 7.42-7.50(m, 2H, ArH), 7.58(d , J=8.4Hz, 1H, ArH), 7.67-7.78(m, 3H, ArH),8.14(d, J=8.4Hz, 1H, ArH); MS (ESI) m / z : 406.1[M+H] + .
Embodiment 2
[0067] Example 2: Synthesis of derivative b, i.e. (E)-2-(2,4-dihydroxyphenyl)vinyl-8-hydroxyquinoline
[0068] N 2 Add 810mg (2.00mmol) intermediate a and 15ml pyridine to a protected 100ml three-neck flask, dissolve, heat to reflux for 1 hour, add 5ml of distilled water, continue to reflux for 11 hours, cool, add 100ml of water, and use n-butanol (50ml×3 ), the organic layer was washed with distilled water (30 mL×2), washed with 30 mL of saturated brine, and the n-butanol layer was dried over anhydrous sodium sulfate overnight. Filtrate and evaporate the solvent under reduced pressure to obtain a crude product, which is separated by column chromatography, and the eluent is: chloroform / methanol ( V:V =9:1) to obtain 496 mg of reddish-brown derivative b with a yield of 89.0%. m.p. greater than 195-197°C; 1 HNMR (400MHz, CD 3 OD) δ: 6.34-6.38(m, 2H, ArH), 7.05(dd, J=7.2Hz, 1H, ArH), 7.26-7.34(m, 3H, ArH), 7.50(d, J=8.4Hz, 1H , ArH), 7.71(d, J=8.4Hz, 1H, ArH), 8.00(d, J=16....
Embodiment 3
[0070] The preparation method of various reagents in the analytical method of the present invention is:
[0071] (1) Preparation method of derivative b solution: weigh 2.8 mg of derivative b, dissolve in acetonitrile, and prepare a 100 mL solution with a concentration of 100 μmol L -1 .
[0072] (2)Al 3+ Standard solution: weigh analytically pure Al(NO 3 ) 3 9H 2 O 75.0mg, dissolved in twice distilled water, and prepared into 100mL solution, Al 3+ The concentration is 2.00×10 -3 mol L -1 ; Dilute step by step with double distilled water to a suitable concentration as required;
[0073] (3) Fe 3+ Standard solution: weigh analytically pure Fe(NO 3 ) 3 9H 2 O 80.8mg, dissolved in double distilled water, and prepared into 100mL solution, Fe 3+ The concentration is 2.00×10 -3 mol L -1 ; Dilute step by step with twice distilled water to a suitable concentration as needed.
[0074] (4) Preparation of other coexisting ionic solutions: Take analytically pure nitrates...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com