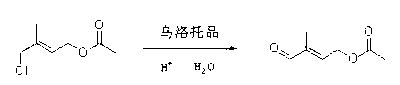Method for preparing 4-acetoxy-2-methyl-2-butenal
A technology of acetoxy and crotonaldehyde, which is applied in the field of organic chemistry, can solve the problems of catalyst TEMPO not being recovered and solvent DMF being difficult to recover, etc., and achieve the effects of avoiding pollution problems, saving raw material costs, and saving environmental protection costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
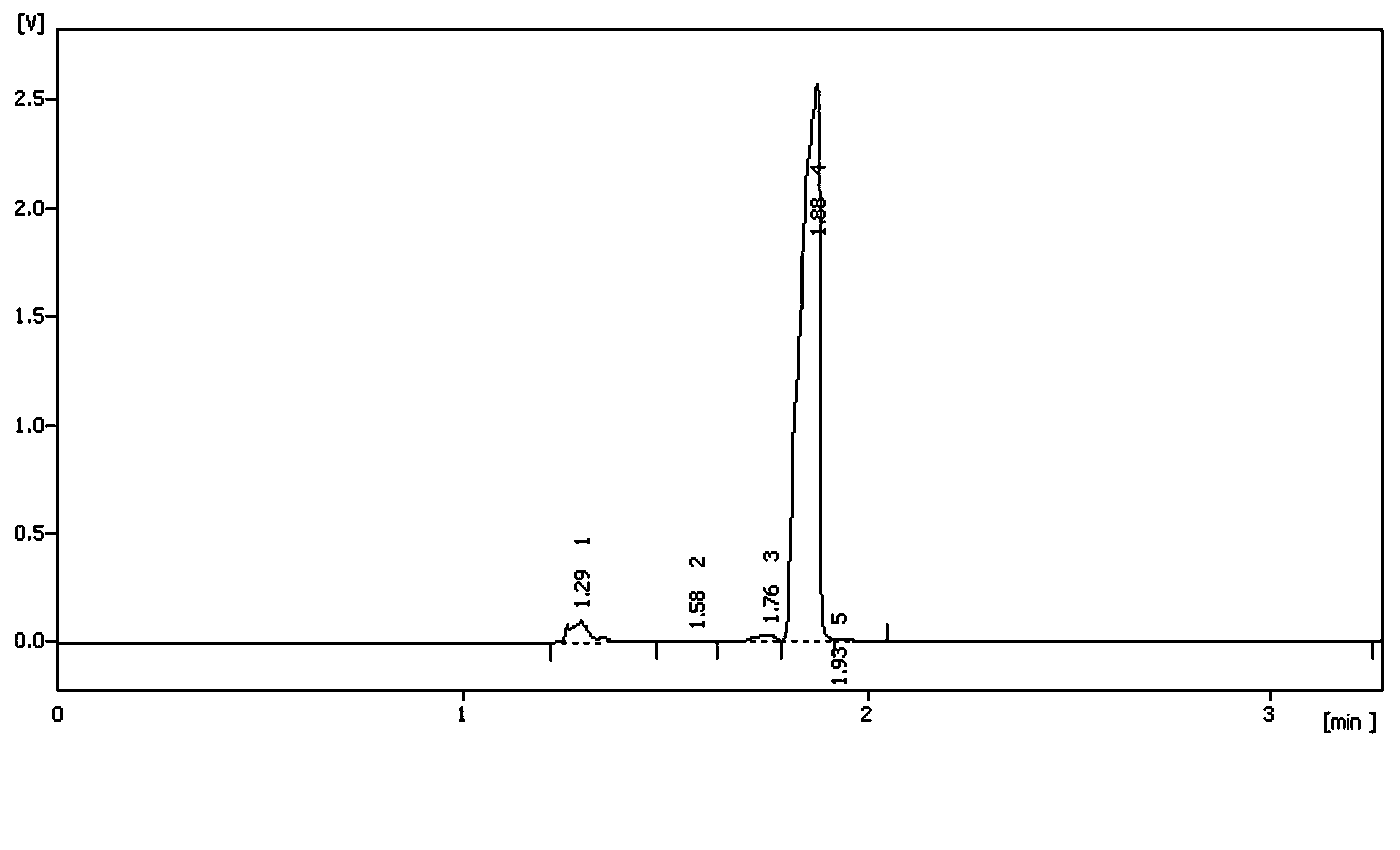
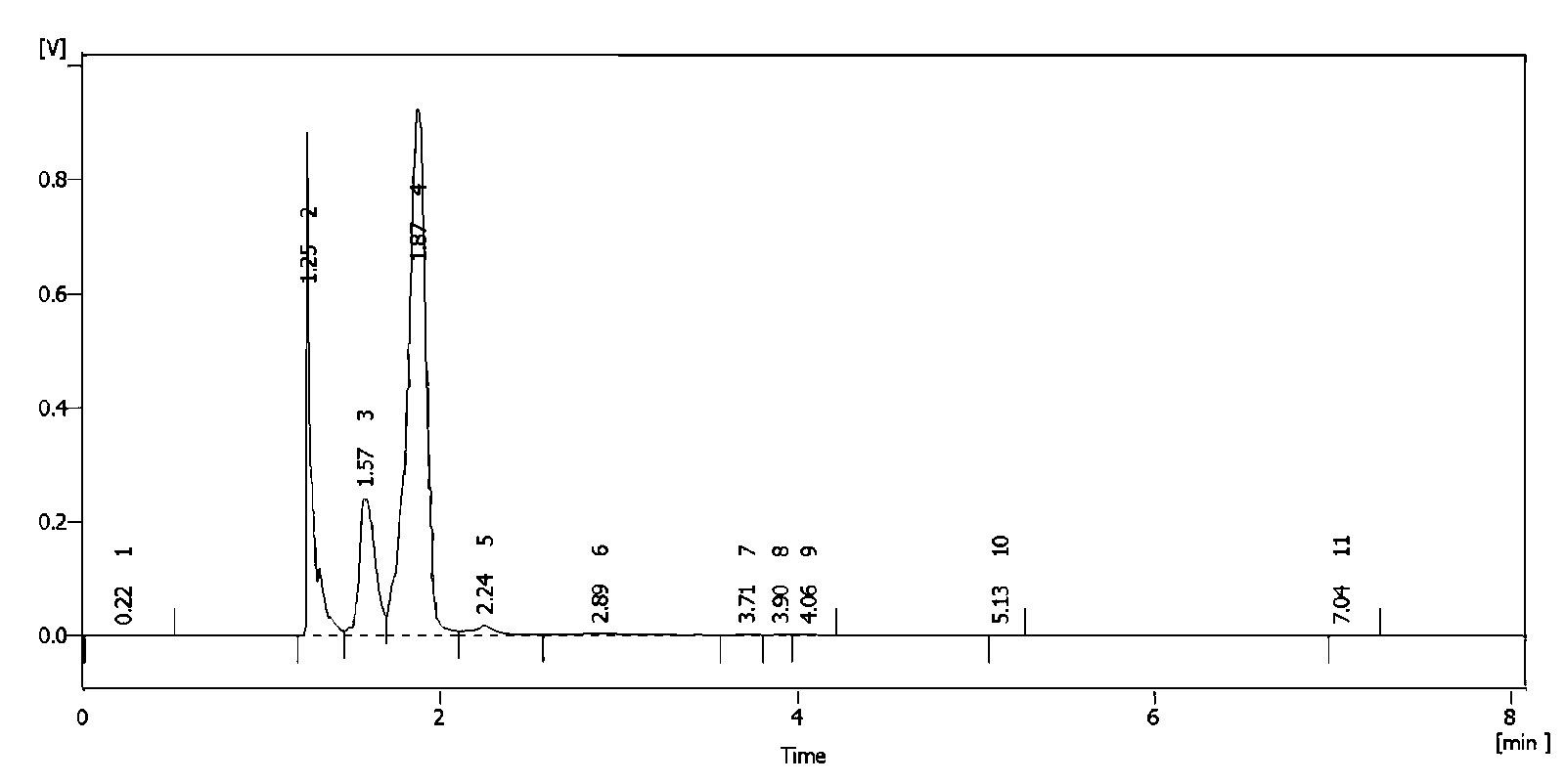
Image
Examples
Embodiment 1
[0050] Dissolve 6.8g of isoprene (0.1mol) in a mixture of 12.0g of acetic acid and 10.2g of acetic anhydride, stir, cool to 15°C, mix tert-butyl hypochlorite with the amount of isoprene and other substances (14.0g, 77%, 0.1mol) was slowly added dropwise and kept at a constant temperature. After the dropwise addition was completed, after detecting that there was no peroxide with starch potassium iodide test paper, 1.7g of p-toluenesulfonic acid (0.01mol) was added and the temperature was raised to After 10 hours of reaction at 60°C, the acetic acid solution containing tert-butanol was recovered by distillation under reduced pressure (can be separated and recovered by rectification). % sodium bicarbonate washed to neutrality, concentrated under reduced pressure, and finally under the pressure of 5mmHg, distilled to obtain chloroester (1-acetoxy-4-chloro-3-methyl-2-butene) 14.0g, Content 93%, yield 80.1%.
[0051] Add 18.1g chlorate (93%, 0.1mol) to 50mL acetone, add 12.6g potas...
Embodiment 2
[0055] Dissolve 68g of isoprene (1mol) in a mixed solution of 120g of acetic acid and 102g of acetic anhydride, stir, cool to 15°C, mix tert-butyl hypochlorite (140.3g, 77%, 1mol) was slowly added dropwise and kept constant temperature. After the dropwise addition was completed, use starch potassium iodide test paper to detect the absence of peroxide, then add 17g of p-toluenesulfonic acid (0.1mol), raise the temperature to 60°C, and react for 10 hours Finally, tert-butanol and acetic acid were recovered by distillation under reduced pressure (separation and recovery by rectification), 200g of ice water was added, and the product chlorine ester was extracted from ice water three times with 600mL of n-hexane, washed with 5% sodium bicarbonate until neutral , concentrated under reduced pressure, and finally under a pressure of 5mmHg, distilled to obtain 144g of chloroester, with a content of 93% and a yield of 82.4%.
[0056] Add 144g chloroester (93%, 0.82mol) to 500mL acetone,...
Embodiment 3
[0060] The method for preparing chloroesters in Example 1 was repeated to obtain 14.3g chloroesters with a purity of 93% and a yield of 81.8%.
[0061] Add 18.0g of chloroester (93%, 0.1mol) into 50mL of acetonitrile, add 12.6g of potassium formate (0.15mol), and 1.61g of n-butylammonium bromide (0.005mol), heat up to reflux, react for 7 hours, cool to 0°C, filtered, distilled to recover the solvent, added 50 mL of ethyl acetate, washed once with water, and concentrated to obtain 18.1 g of diester with a content of 91% and a yield of 95.8%.
[0062] Add 19g of diester (91%, 0.11mol) into 50mL of methanol, add 0.42g of sodium acetate (0.005mol), react at room temperature for 3 hours, recover methanol by distillation under reduced pressure, add 50mL of ethyl acetate to dissolve, filter off sodium acetate After concentration, 17.2 g of alcohol ester was obtained, with a content of 90% and a yield of 97.7%.
[0063] Dissolve 16g of alcohol ester (91%, 0.11mol) in 50mL of acetonit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com