Arctigenin pro-drug, preparation method and use thereof
A technology of arctigenin and prodrug, which is applied in the field of water-soluble arctigenin prodrugs, can solve the problems such as the inability to significantly improve oral absorption, and achieves the expansion of the route of administration, the improvement of oral bioavailability, and the good water solubility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0025] Preparation example 1 prepares arctigenin
[0026] After crushing 500g burdock fruit, reflux extraction with 4 liters of 70% ethanol water for 2 times, each time for 2 hours, after recovering ethanol from the extract, apply it to a macroporous adsorption resin, elute with 95% ethanol, 50% ethanol, and 30% ethanol, and collect 50% ethanol The solvent was eluted, and the solvent was recovered to obtain 43 g of the composition. Through silica gel column chromatography 30: 1, ethyl acetate: absolute ethanol (8: 1) was used as the eluent to elute to obtain 20 g of arctiin, which was dissolved in 50% ethanol was hydrolyzed with 4% hydrochloric acid, and the ethanol was recovered. The solution was white and turbid. It was extracted three times with an equal volume of chloroform, the chloroform layers were combined, and the solvent was recovered to obtain 14.4 g of arctigenin. The purity measured by HPLC was 98.2%. .
Embodiment 1
[0027] Embodiment 1 prepares arctigenin succinate monoester
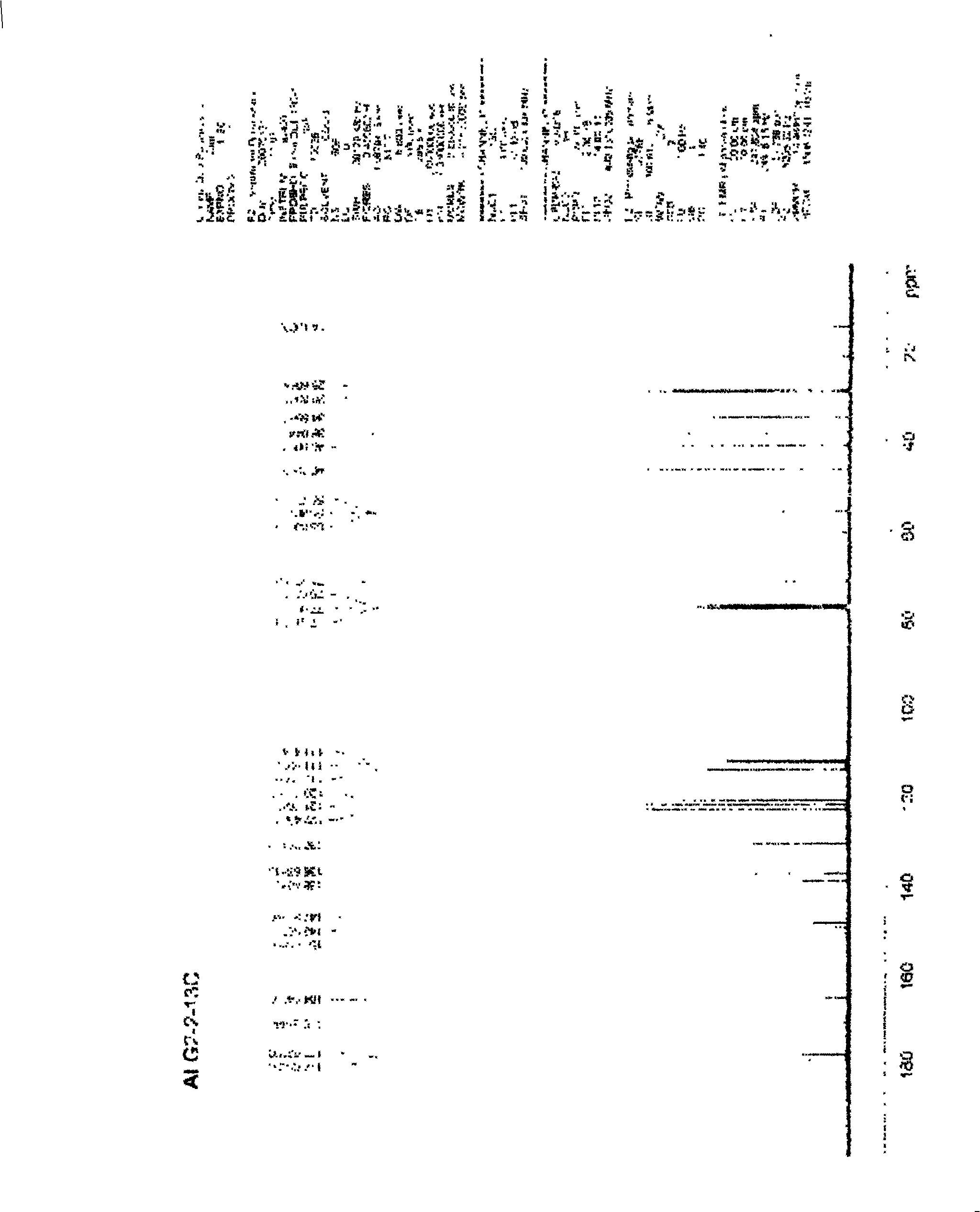
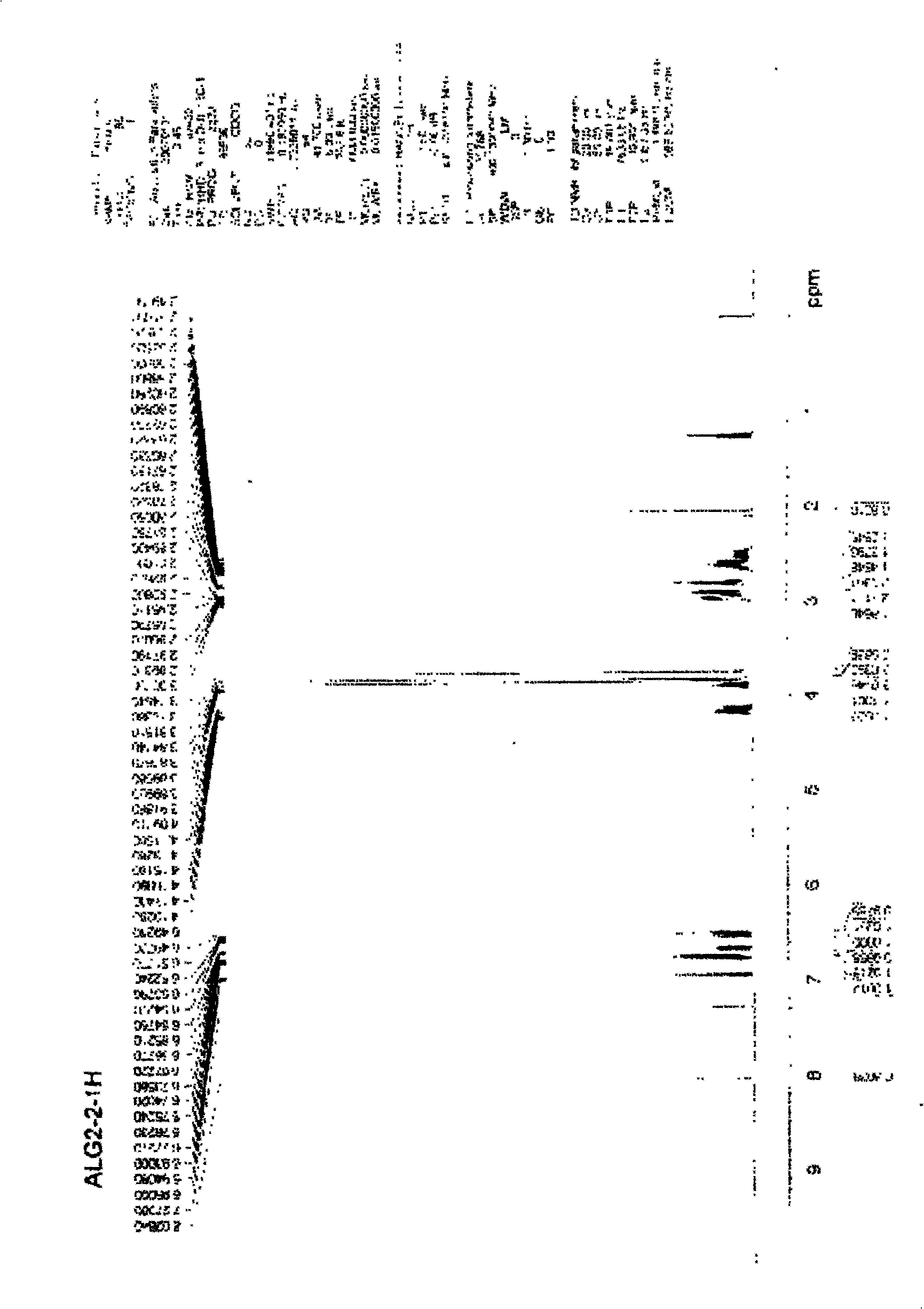
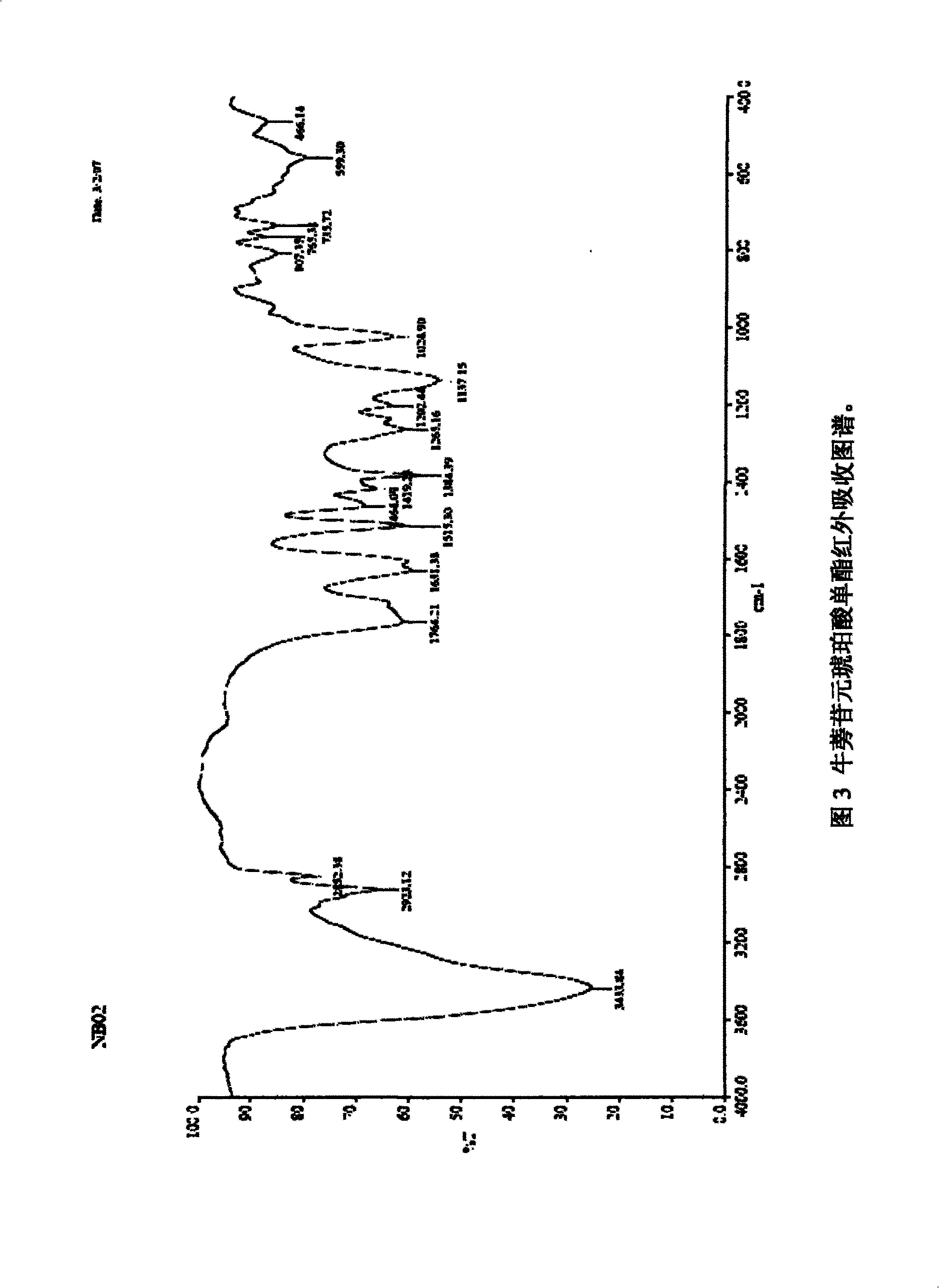
[0028] A certain amount of arctigenin is first dissolved in 25ml of anhydrous dichloromethane, and another round-bottomed flask with a suitable volume is taken, and 1.5 times the succinic anhydride of arctigenin is weighed, injected into 25ml of anhydrous dichloromethane, and the , drop the above-prepared raw material solution into the succinic anhydride solution under stirring conditions, stir for 3 to 7 days, stop the reaction, add an appropriate amount of hydrochloric acid solution, add ethyl acetate for extraction, combine the extracts, and successively use a small amount of Wash with water and saturated brine, dry the ethyl acetate layer in anhydrous sodium sulfate for 2 hours, concentrate under reduced pressure to obtain extract, silica gel column chromatography, petroleum ether ~ ethyl acetate ~ formic acid system [75:24:1] as the washing Remove the agent, concentrate and dry to obtain arctigenin succinic aci...
Embodiment 2
[0036] Embodiment 2: Preparation of Arctigenin Succinate Monoester Tablets
[0037]Weigh 10g of arctigenin succinic acid monoester, mix well, pass through 80 mesh sieve, add 200g of microcrystalline cellulose and 90g of starch as diluent to form the preparation formula, mix well, spray 75% ethanol as binder to make soft material , granulated with a 24-mesh sieve, granulated after drying, mixed evenly, compressed into tablets, made into 1000 tablets, coated, and obtained, each tablet of arctigenin succinic acid monoester 100mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| water solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com