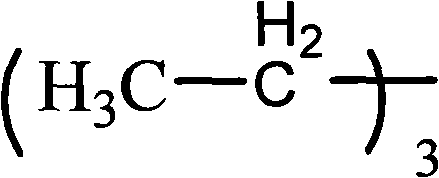Aqueous macromolecular photoinitiator and preparation method thereof
A photoinitiator and macromolecular technology, applied in the field of water-based macromolecular photoinitiator and its synthesis, to achieve good photoinitiation efficiency, reduce production costs, and increase content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
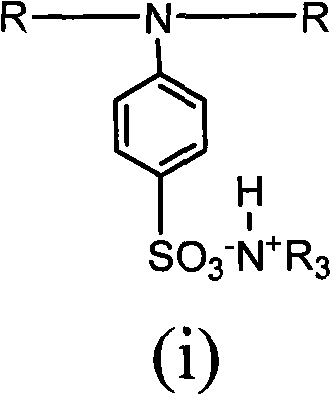
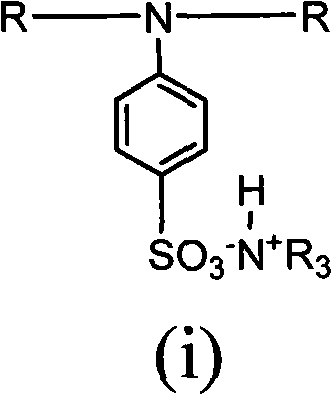
[0034] Example 1: Add 8.50 g of 2-hydroxyl-1-{4-[4-(2-hydroxyl-2-methyl-propionyl)-benzyl to a three-necked flask equipped with a thermometer, reflux condenser, and mechanical stirring ]-phenyl}-2-methyl-propane, 23.31g isophorone diisocyanate and 20.00ml acetone, in the 2 Under conditions, 80°C, react for 3 hours; then add 2.92g dimethylol propionic acid, gradually cool down to 75°C, react for 2 hours, add 21.60g polypropylene glycol, 1.34g ethylene glycol, gradually heat up to 80°C, react When the isocyanate group reaches the theoretical value, cool down to 50°C, add 2.20g of triethylamine, neutralize, and set aside; add 0.87g of 4-aminobenzenesulfonic acid to another three-necked flask equipped with a thermometer, reflux condenser, and mechanical stirring , 100g deionized water, heated to 70°C, added prepolymer, reacted until the isocyanate group disappeared completely, added triethylamine to neutralize, adjusted PH=8~9, distilled off acetone, and obtained a water-based sol...
Embodiment 2
[0035] Embodiment two: add 8.50g 2-hydroxyl-1-{4-[4-(2-hydroxyl-2-methyl-propionyl)-benzyl to a three-necked flask equipped with a thermometer, a reflux condenser, and mechanical stirring ]-phenyl}-2-methyl-propane, 18.27g toluene diisocyanate and 20.00ml acetone, in general N 2 Under the conditions, 80°C, react for 3 hours; then add 3.75g dimethylol propionic acid, gradually cool down to 75°C, react for 2 hours, add 18.50g polypropylene glycol, 1.92g neopentyl glycol, gradually heat up to 80°C, React until the isocyanate group reaches the theoretical value, lower the temperature to 50°C, add 2.83g of triethylamine, neutralize, and set aside; add 0.87g of 4-aminobenzenesulfonate to another three-necked flask equipped with a thermometer, reflux condenser, and mechanical stirring Acid, 100g deionized water, heated to 70°C, added prepolymer, reacted until the isocyanate group disappeared completely, added triethylamine to neutralize, adjusted PH=8~9, distilled off acetone to obta...
Embodiment 3
[0036] Embodiment three: add 8.50g 2-hydroxyl-1-{4-[4-(2-hydroxyl-2-methyl-propionyl)-benzyl in a three-necked flask equipped with a thermometer, a reflux condenser, and mechanical stirring ]-phenyl}-2-methyl-propane, 17.01g of hexamethylene diisocyanate and 20.00ml of acetone, in general N 2Under the condition of 80°C, react for 3 hours; then add 3.11g of dimethylolpropionic acid, gradually cool down to 75°C, react for 2 hours, add 20.90g of polypropylene glycol, 1.59g of 1,3-propanediol, and gradually heat up to 80°C, React until the isocyanate group reaches the theoretical value, lower the temperature to 50°C, add 2.34g of triethylamine, neutralize, and set aside; add 0.87g of 4-aminobenzenesulfonate to another three-necked flask equipped with a thermometer, reflux condenser, and mechanical stirring Acid, 100g deionized water, heated to 70°C, added prepolymer, reacted until the isocyanate group disappeared completely, added triethylamine to neutralize, adjusted PH=8~9, dist...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com