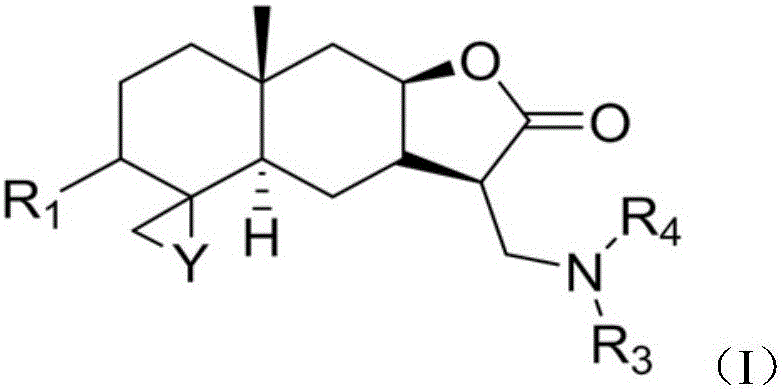
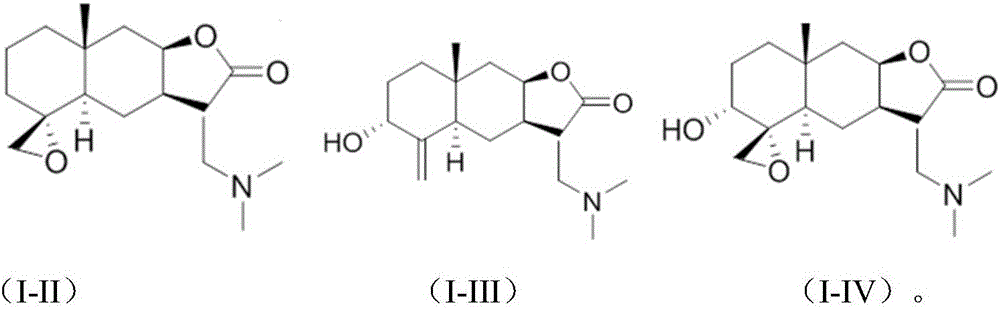
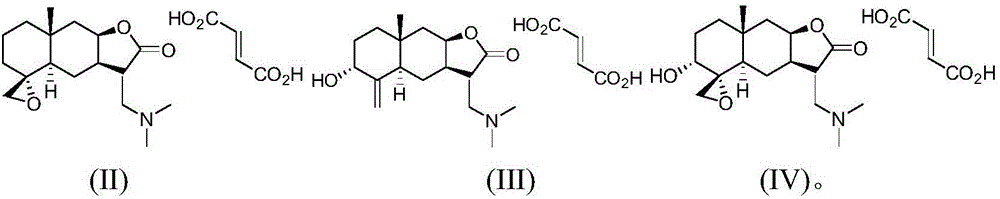
Isoalantolactone derivative and salt thereof
A kind of technology of xerosalactone and its derivatives, which is applied in the direction of drug combination, carboxylate preparation, endocrine system diseases, etc., can solve the problems of poor water solubility, low bioavailability, short half-life, etc., and achieve improved survival, high The effect of tumor inhibition rate and excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of compound (II)
[0029]
[0030] 1) Preparation of compound (1.1)
[0031]
[0032] 1) Isoaurinolide (1.0g, 4.3mmol) was dissolved in dichloromethane (16mL), and peroxybenzoic acid (1.1g, 5.2mmol) was slowly added to the system in batches, reacted at room temperature for 3 hours, saturated The reaction was quenched with sodium thiosulfate (16 mL), extracted with dichloromethane (16 mL×3), the organic phase was washed with saturated aqueous sodium bicarbonate (16 mL), dried and concentrated, and purified by column chromatography (petroleum ether: ethyl acetate=20: 1) Obtain compound 1.2 (white solid, 984 mg, yield 92.0%).
[0033] 2) preparation of compound (I-II),
[0034]
[0035] The compound 1.2 obtained in step 1) was dissolved in dichloromethane (98mL), potassium carbonate (16.4g, 118.9mmol) and dimethylamine hydrochloride (4.8g, 59.4mmol) were added to the system in turn, and the mixed system was stirred Reflux reaction for 3 hours, after...
Embodiment 2
[0041] Preparation of compound (III)
[0042]
[0043] 1) Preparation of compound 1.3
[0044]
[0045] At 0°C, dissolve selenium dioxide (87.5mg, 0.75mmol) in dichloromethane (5mL), add tert-butanol peroxide (0.37mL), and stir for 30 minutes. , 2.15mmol) of dichloromethane (5mL) solution was slowly added to the above system, stirred at room temperature for 24 hours, quenched with saturated aqueous sodium thiosulfate (8mL), extracted with dichloromethane (8mL×3), dried Concentrate and purify by column chromatography (petroleum ether: ethyl acetate = 9:1 to 3:1) to obtain compound 1.23 (white solid, 350 mg, yield: 82.8%).
[0046] 2) Preparation of compound (I-III)
[0047]
[0048] The compound 1.3 obtained in the previous step was dissolved in dichloromethane (35mL), potassium carbonate (5.8g, 42.2mmol) and dimethylamine hydrochloride (1.7g, 21.1mmol) were added to the system successively, and the mixed system was stirred and refluxed Reacted for 3 hours. After th...
Embodiment 3
[0053] Preparation of Compound IV
[0054]
[0055] 1) Preparation of compound 1.4
[0056]
[0057] Compound 1.3 (112mg, 0.45mmol) was dissolved in dichloromethane (1mL), and a solution of peroxybenzoic acid (100mg, 0.60mmol) in dichloromethane (1mL) was slowly added dropwise to the above system, and reacted at room temperature for 2 hours , quenched the reaction with saturated sodium thiosulfate (2 mL), extracted with dichloromethane (2 mL×3), dried and concentrated, and purified by column chromatography (petroleum ether: ethyl acetate = 4:1 to 1:1) to obtain compound 1.4 ( White solid, 89 mg, yield: 74.5%).
[0058] 2) Preparation of compound (I-IV)
[0059]
[0060] The compound 1.4 obtained in the previous step was dissolved in dichloromethane (35mL), potassium carbonate (5.8g, 42.2mmol) and dimethylamine hydrochloride (1.7g, 21.1mmol) were added to the system successively, and the mixed system was stirred and refluxed Reacted for 3 hours. After the reaction, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com