Chemical synthesis method of laetispicine
A technology of quincetin and chemical synthesis, which can be applied in the fields of organic chemistry, drug combination, nervous system diseases, etc., and can solve the problems of unfavorable structural modification research and low content of quincetin.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
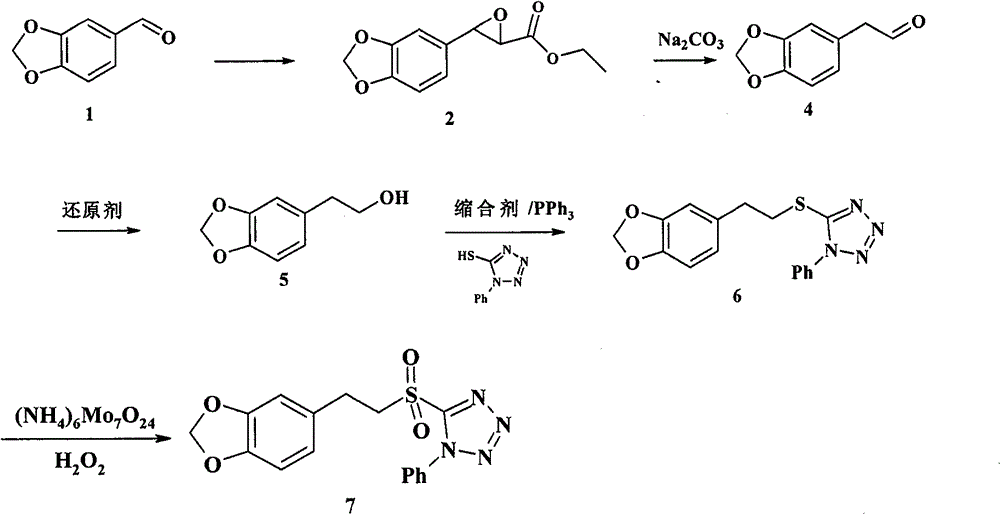
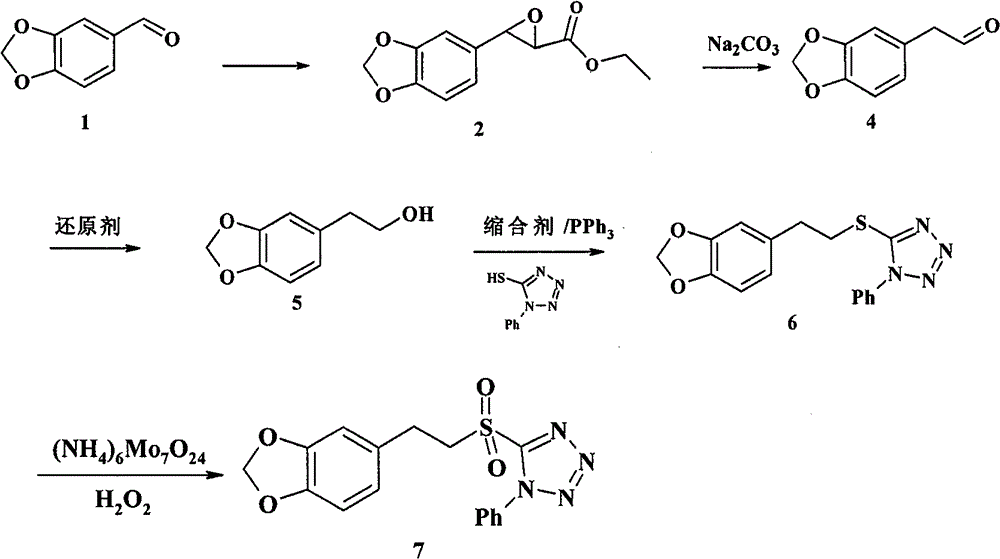
[0034] Example 1 The preparation of 3,4-methylenedioxyphenyl glycidic acid methyl ester (2)
[0035] Dissolve 6.0g (40mmol) of piperonal (1) and 4.6ml (60mmol) of methyl chloroformate in 15ml of absolute methanol, slowly drop into 5ml of absolute methanol containing 6.5g (60mmol) of sodium methoxide in an ice-salt bath under the protection of argon solution, the dropwise addition time was about 10 minutes, and after the dropwise addition was completed, it was stirred in an ice-salt bath for 1 hour. Add a mixed solution of 20ml water and 20ml diethyl ether to the reaction solution, stir in an ice bath for 1h, a large amount of solids are generated, filter with suction, wash with diethyl ether, and dry to obtain white solid 3,4-methylenedioxyphenylmethyl glycidate (2 ) 7.8g, crude product yield 88%, directly carry out next step reaction. Wherein, the methyl chloroformate and sodium methoxide can also be replaced by ethyl chloroacetate and sodium ethoxide, and the reaction solve...
Embodiment 2
[0037] Example 2 Preparation of 3,4-methylenedioxyphenylacetaldehyde (4)
[0038]Dissolve 14.0g of sodium methoxide in 100ml of absolute methanol, slowly drop into 56.6g (0.25mol) (2) of 50ml of anhydrous toluene solution under the protection of argon in an ice bath, stir in an ice bath for 30 minutes, add 6ml of water and 200ml of Diethyl ether was stirred in an ice bath for 2 hours, a large amount of solids were produced, suction filtered, washed with anhydrous ether, and dried to obtain 64.9 g of crude sodium 3,4-methylenedioxyphenyl glycidic acid, which was directly carried out to the next reaction without further treatment.
[0039] Dissolve 64.9g (0.28mol) of crude product 3,4-methylenedioxyphenyl glycidic acid sodium and 16.2ml (0.28mol) of glacial acetic acid in 50ml of anhydrous toluene solution, heat and reflux for 2.5 hours (a large amount of CO 2 After cooling, the organic phase was washed twice with 2×50 ml of water, dried over anhydrous sodium sulfate, filtered, ...
Embodiment 3
[0041] Example 3 Preparation of 3,4-methylenedioxyphenethyl alcohol (5)
[0042] Under ice-cooling, dissolve 0.30g (1.8mmol) (4) in 15ml of anhydrous methanol, slowly add dropwise a solution of 0.09g (2.2mmol) sodium borohydride in 5ml of anhydrous methanol, and stir in ice-bath for 15 minutes. Add 20ml of water and 20ml of dichloromethane, wash the aqueous phase twice with 2×20ml of dichloromethane, combine the organic phases, dry over anhydrous sodium sulfate, filter, and evaporate the solvent to obtain a colorless oily substance 3,4-methylenedioxy Phenylethyl alcohol (5) 0.28g, yield 93%. Proceed directly to the next reaction.
[0043] 1 H-NMR (CDCl 3 , 300MHz) δ: 2.79 (t, 2H), 3.83 (t, 3H), 5.94 (s, 2H), 6.67-6.78 (m, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com