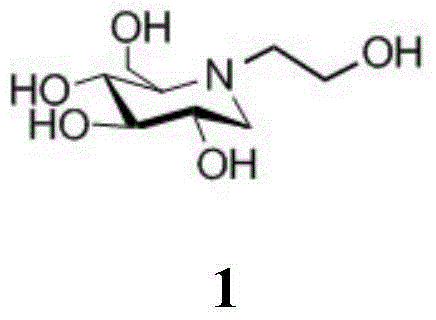
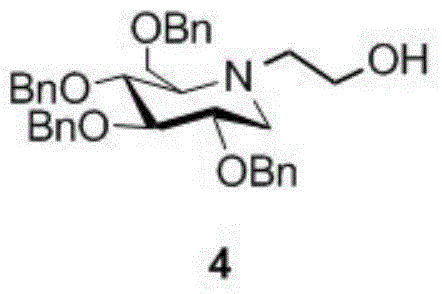
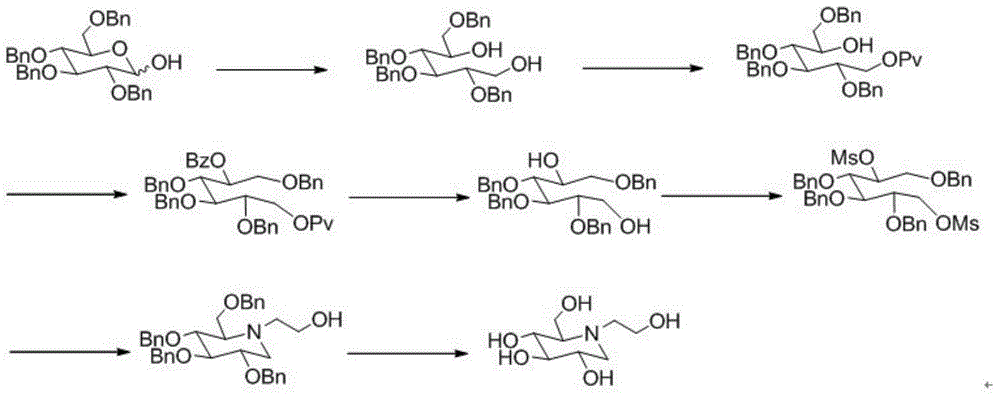
A preparing method of a miglitol intermediate
A technology of tetrabenzyl miglitol and diol, which is applied in the field of preparation of miglitol intermediates, can solve the problems of low yield, complicated operation, and no preparation method of key intermediate N-substituted glucosamine , to achieve the effect of short reaction time, high yield, and omission of separation and purification steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Preparation of (2,3,4,6)-O-tetrabenzylglucodiol (2)
[0036] Add 54.00g (100mmol) of tetrabenzylglucose, 10.10g (260mmol) of sodium borohydride, and 500mL of absolute ethanol into a 1L reaction flask, react for 24h under room temperature stirring, and concentrate the reaction solution under reduced pressure. After adding 100 mL of ethyl acetate to the residue, wash with water, and wash the organic phase with anhydrous Mg 2 SO 4 After drying, filtering and concentrating, 53.10 g of a colorless, transparent viscous object was obtained. The purity as determined by HPLC was 96.0%, and the yield was 98.0%.
[0037] 1 H NMR (400MHz, CDCl 3 )δ7.41–7.19(m,20H),4.74–4.47(m,8H),4.08–3.99(m,1H),3.88(dt,J=7.0,3.6Hz,1H),3.82–3.69(m, 3H),3.67–3.60(m,2H),3.57(dd,J=11.8,4.4Hz,1H),2.89(dd,J=24.5,3.4Hz,1H),2.17–1.88(br,1H).
Embodiment 2
[0038] Example 2: Preparation of Tetrabenzyl Miglitol (4)
[0039] (1) Preparation of Tetrabenzyl Glucose Dicarbonyl Derivatives (3)
[0040] Add 8.0mL (93mmol) oxalyl chloride and 80mL dichloromethane into a 250mL reaction flask, and cool down to -78°C. A mixture of 7.1 mL (100 mmol) of dimethyl sulfoxide and 50 mL of dichloromethane was added dropwise with stirring, and stirred at -78°C for 40 min. Dissolve 10.8g (20mmol) of tetrabenzyl glucodiol in 40mL of dichloromethane and slowly drop into the above system, react at -65°C for 2.5h. Add 33.2 mL of triethylamine dropwise to the reaction system, slowly raise the temperature to 0°C and stir for 20 min, and the oxidation reaction solution obtained after the reaction is directly put into the next reaction without treatment.
[0041] (2) Preparation of Tetrabenzyl Miglitol (4)
[0042] Add 4.80 g (80 mmol) of ethanolamine, 5.00 g (80 mmol) of sodium cyanoborohydride, and 500 mL of methanol into a 1000 mL reaction flask, and ...
Embodiment 3
[0045] Example 3: Preparation of Tetrabenzyl Miglitol (4)
[0046] (1) Preparation of Tetrabenzyl Glucose Dicarbonyl Derivatives (3)
[0047] Add 8.0mL (93mmol) oxalyl chloride and 80mL dichloromethane into a 250mL reaction flask, cool down to -70°C, slowly add a mixture of 7.1mL (100mmol) dimethyl sulfoxide and 50mL dichloromethane dropwise under stirring, at -70°C Stir for 40min. Dissolve 10.80g (20mmol) of tetrabenzyl glucodiol in 40mL of dichloromethane and add dropwise to the above system, and react at -70°C for 2.5h. Add 33.2 mL of triethylamine dropwise to the reaction system, slowly raise the temperature to 10°C and stir for 20 min, and the oxidation reaction solution obtained after the reaction is directly put into the next reaction without treatment.
[0048] (2) Preparation of Tetrabenzyl Miglitol (4)
[0049] Add 2.40 g (40 mmol) of ethanolamine, 2.50 g (40 mmol) of sodium cyanoborohydride, and 500 mL of methanol into a 1000 mL reaction flask, and stir at room t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com