Method for directly synthesizing cyclic carbonate from olefin under catalysis of metalloporphyrin
A technology of porphyrin catalyzing olefins and cyclic carbonates, applied in chemical instruments and methods, catalytic reactions, catalysts for physical/chemical processes, etc., can solve problems such as low yields, etc. The effect of process integration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
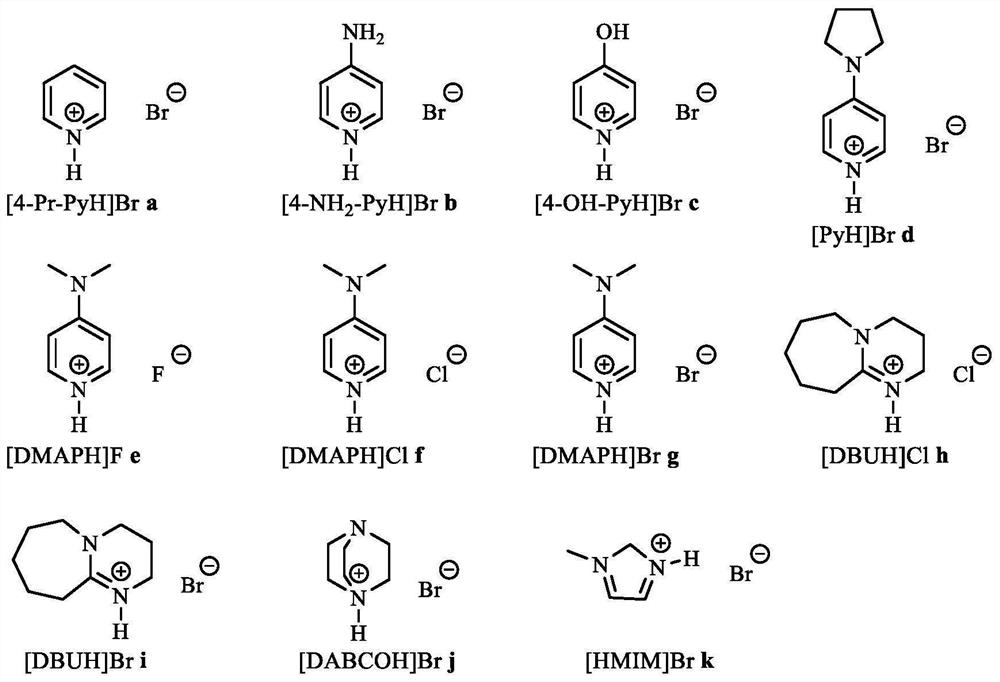
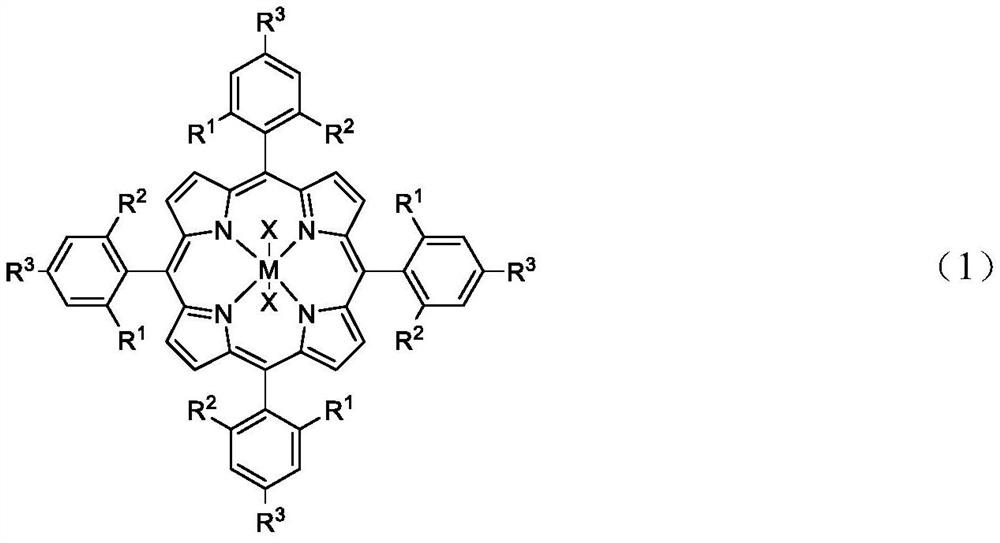
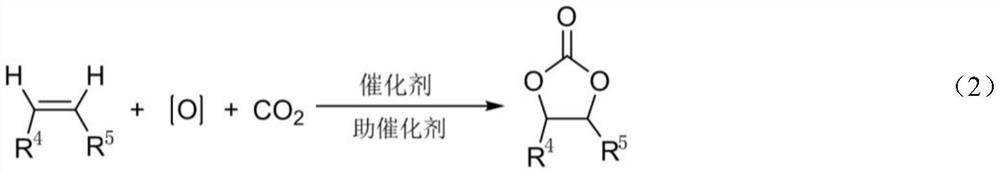
[0032] Styrene (1.2g, 10mmol), manganese tetraphenylporphyrin (1.0mg, 0.14%mmol), cocatalyst [4-Pr-PyH]Bra (40mg, 0.25%mol), 0.1MPa oxygen and 1.2MPa carbon dioxide , reacted at 80°C for 8h, stopped the reaction to obtain the corresponding cyclocarbonate, and its yield was 89% by gas phase detection.
Embodiment 2
[0034] Styrene (1.2g, 10mmol), manganese tetraphenylporphyrin (1.0mg, 0.14%mmol), cocatalyst [4-NH 2 -PyH]Br b (44 mg, 0.25% mol), the reactor was closed, and 0.8 MPa oxygen and 1.2 MPa carbon dioxide were introduced. React at 100° C. for 8 h, stop the reaction to obtain the corresponding cyclic carbonate, and its yield is 95% by gas phase detection.
Embodiment 3
[0036] In a 100mL autoclave, add styrene (1.2g, 10mmol), manganese tetraphenylporphyrin (1.0mg, 0.14%mmol), cocatalyst [4-OH-PyH]Br c (44mg, 0.25%mol ), close the reactor, and feed 0.6MPa oxygen and 1.2MPa carbon dioxide. After reacting at 130°C for 8 hours, the reaction was stopped to obtain the corresponding cyclocarbonate, and the yield was 90% by gas phase detection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com