Synthesizing method of 1-benzyl-piperidone hydrochloride
A technology of piperidone hydrochloride and synthesis method, which is applied in the field of synthesis of 1-benzyl-3-piperidone hydrochloride, and can solve the problems of reducing hydrogen pressure, difficult cost reduction, and high cost of precious metal catalysts , to achieve the effects of short steps, cost reduction and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
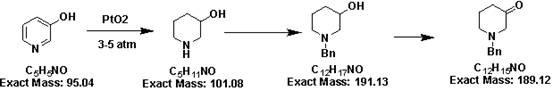
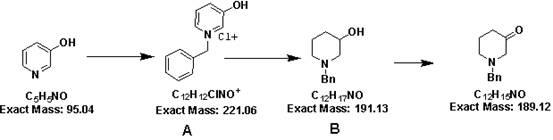
[0034] The synthetic method of 1-benzyl-3-piperidone hydrochloride comprises the steps:
[0035]
[0036] (1) Weigh 30 grams (0.32mol) of the raw material 3-hydroxypyridine, add 400ml of toluene, heat to dissolve, and under reflux, add 40 (0.32mol) benzyl chloride dropwise, finish adding within 30 minutes, and continue to reflux React for 2 hours. TLC detects that the reaction is complete. After the reaction was cooled to room temperature, it was suction filtered and dried to obtain 65 g of product A, with a yield of 94%.
[0037]
[0038] (2) Add 65 g (0.29 mol) of the product A prepared in the first step to 800 ml of ethanol, slowly add 22.33 (0.59 mol) of sodium borohydride in batches under ice bath, and return to room temperature and stir for 10-15 Hour. As detected by TLC, the reaction was complete, and 200 ml of water was added to quench the reaction solution. Spin off the ethanol, add concentrated hydrochloric acid to adjust the pH to 1-2, extract impurities t...
Embodiment 2
[0042] The synthetic method of 1-benzyl-3-piperidone hydrochloride comprises the steps:
[0043]
[0044] (1) Weigh 300 (3.2mol) grams of raw material 3-hydroxypyridine, add 5000ml of toluene, heat to dissolve, under reflux, add 390g (3.08mol) of benzyl chloride dropwise, finish adding within 60 minutes, and continue to reflux React for 2 hours. TLC detects that the reaction is complete. After the reaction was cooled to room temperature, it was suction filtered and dried to obtain 630 g of product A with a yield of 90%.
[0045]
[0046] (2) Add 630 g (2.85 mol) of the product A prepared in the first step to 8000 ml of ethanol, slowly add 250 (6.6 mol) grams of sodium borohydride in batches under ice bath, return to room temperature and stir for 10-15 Hour. TLC detects that the reaction is complete. Add 2000 ml of water to quench the reaction solution. Spin off the ethanol, add concentrated hydrochloric acid to adjust the pH to 1-2, extract impurities twice with 1000 ...
Embodiment 3
[0050] The synthetic method of 1-benzyl-3-piperidone hydrochloride comprises the steps:
[0051]
[0052] (1) Weigh 1000 g (10.52 mol) of raw material 3-hydroxypyridine, add 5000 ml of toluene, heat to dissolve, and add 1280 g (10.11 mol) of benzyl chloride dropwise under reflux, finish adding within 60 minutes, and continue to reflux React for 2 hours. TLC detects that the reaction is complete. After the reaction was cooled to room temperature, it was suction filtered and dried to obtain 2165 g of product A with a yield of 93%.
[0053]
[0054] (2) Add 2165 g (9.79 mol) of the product A prepared in the first step to 15 liters of ethanol, slowly add 800 g (21.12 mol) of sodium borohydride in batches under ice bath, and return to room temperature and stir for 10-15 Hour. TLC detects that the reaction is complete. Add 2000 ml of water to quench the reaction solution. Spin off the ethanol, add concentrated hydrochloric acid to adjust the pH to 1-2, extract the impurit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com