A kind of preparation method of anticancer drug lanosterol derivative
A technology of lanosterol and anticancer drugs, applied in the field of drug synthesis, can solve the problems of unfavorable industrial production, small amount of synthesis, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
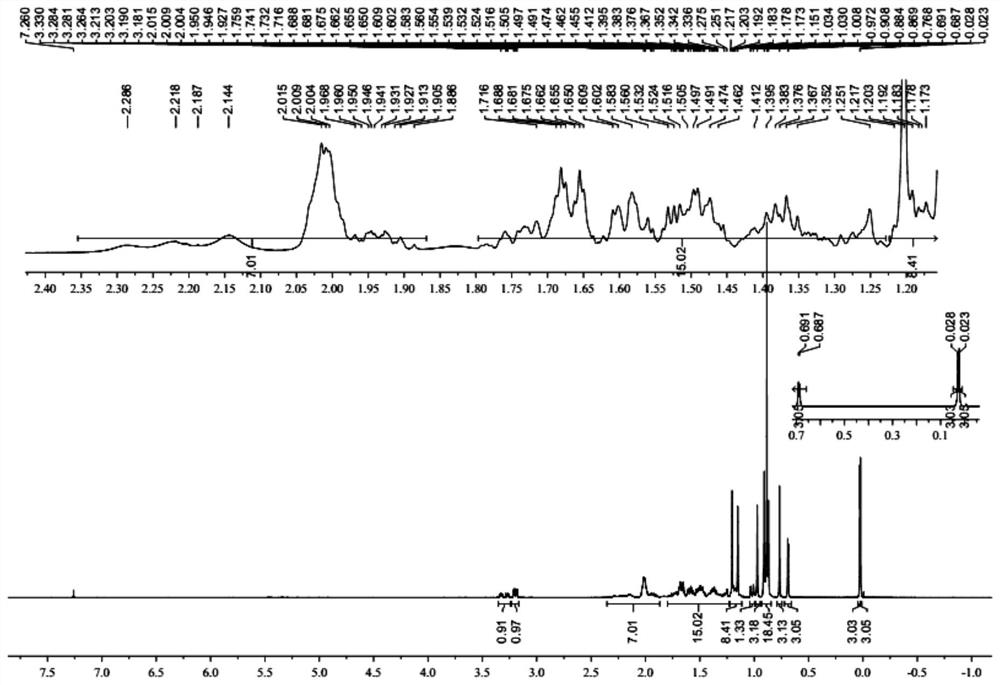
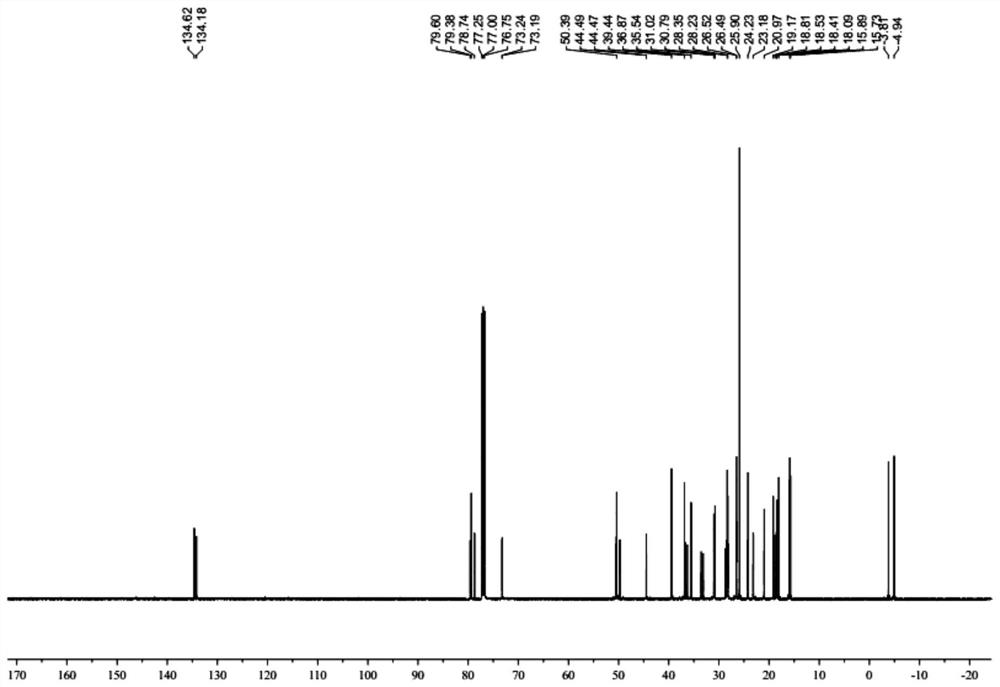
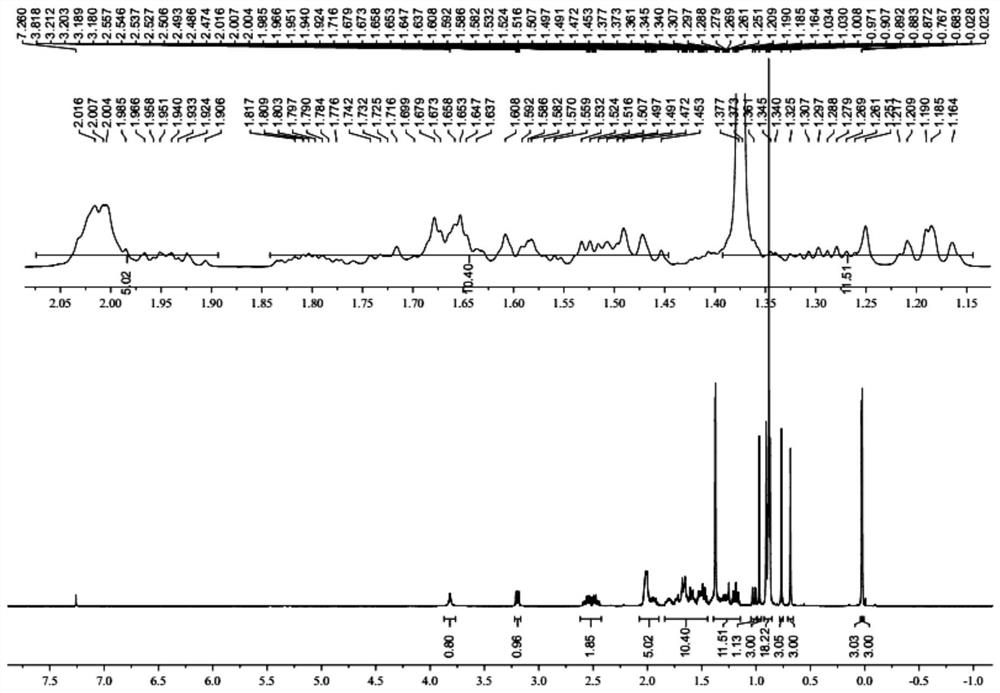
[0044] Example 1: Preparation of lanosterol derivative LD
[0045] Preparation of A, LD-A:
[0046] (1) 300.00 g (0.7 mol) of lambol, 203.40 g (1.9 mol) 2,6-dimethylpyridine, 3000 ml of dichloromethane was added to 5000 ml of reaction flask, and the mixture was stirred. Toned at 20 ° C to 30 ° C, add 371.70 g (1.4 mol) TBSOTF to the reaction liquid, and the dripping process is exothermic, and the reaction temperature does not exceed 40 ° C. After the dropwise addition, 30-40 ° C after 2-3 h, sampling, HPLC monitoring reaction, and the area normalization method showed that sheepterol was less than 0.5%, which judged to the end of the reaction. A 1200 ml of saturated aqueous ammonium chloride solution was added, and after stirring for 10 min, the stratified layer was allowed to collect organic phases. The aqueous phase was extracted twice with dichloromethane (500 mL each time) to discard the water phase. The combined organic phase was washed with 1500 ml of saturated brine, and aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com