Chrysin non-natural amino acid derivative as well as preparation method and application thereof
An unnatural amino acid, chrysin technology, applied in the field of medicine, can solve the problems of poor water solubility, less intestinal absorption, easy metabolic inactivation and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
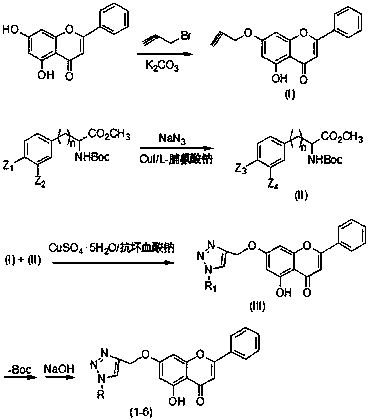
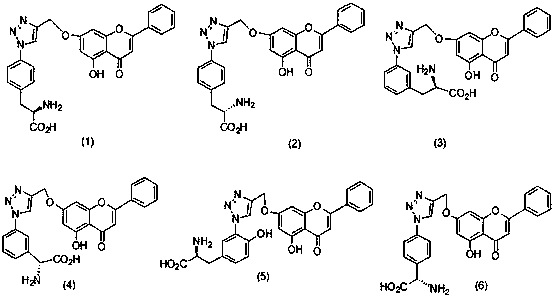
[0025] Preparation of compound (1)
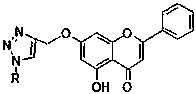
[0026] 2.5g (10 mmol) chrysin and 3.36g (20 mmol) K 2 CO 3 Added to the reactor containing 100mL of anhydrous acetone, slowly added 2 mL of propyne bromide under vigorous stirring, and refluxed for 3h. After the reaction was completed, 50 mL of water was added, acetone was removed under reduced pressure, filtered, the filter cake was washed with water, 1M HCl solution and water in sequence, and dried to obtain a yellow solid, which was compound (I), with a yield of 95%. mp:153.7-154.5℃; 1 HNMR (CDCl 3 ,400MHz)δ:12.72(s,1H), 7.89(d, J =4Hz,2H),7.53(m,3H),6.68(s,1H),6.59(d, J =1.6Hz,1H),6.45(d, J = 2.0 Hz,1H),4.77(d, J = 2.0 Hz, 2H), 2.61(s, 1H); ESI-MS(m / z): 293[M+H] + .
[0027] 3.58g (10mmol) of (L)-4-Br-Boc-PheOMe and 0.78g (12 mmol) of NaN 3 Add 15 mL of DMSO, then add 0.19 g (1 mmol) of CuI and 0.41 g (3 mmol) of L-proline sodium, heat to 90 ° C, react for 5 h, add 50 mL of H 2 O, filter, then add 50mL CH 2 Cl 2 , layered...
Embodiment 2
[0032] Preparation of compound (2)
[0033] (D)-4-Br-Boc-PheOMe was used instead of (L)-4-Br-Boc-PheOMe, and other operations were the same as in Example 1 to obtain compound (2) with a yield of 86%.
Embodiment 3
[0035] Preparation of compound (3)
[0036] (L)-4-Br-Boc-PheOMe was replaced by (L)-3-I-Boc-PheOMe, and other operations were the same as in Example 1 to obtain compound (3) with a yield of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com