Preparation and application of a swern reagent
A reaction and organic solvent technology, applied in the field of new methyl sulfoxide reagents, can solve the problems of cumbersome operation, unresolved reaction temperature, and long time required for the separation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
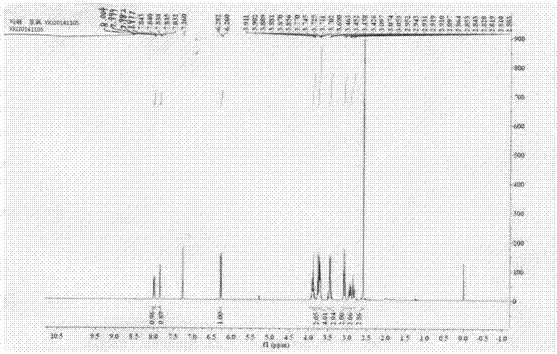
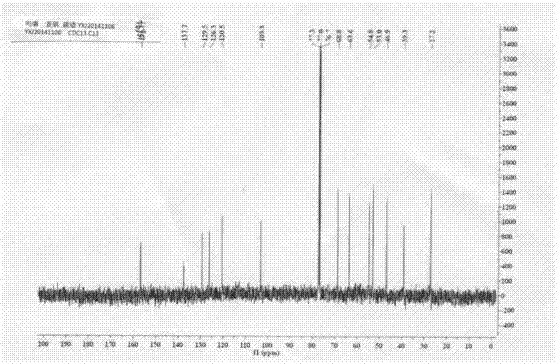
[0055] Example 1: Preparation of 4-(2-(2-(methylsulfoxide) ethyl)-4-nitrophenyl)morpholine (I)
[0056] (1) Add 5.0g (24.79mmol) 2-(2-chloro-5nitro)phenylethanol (II) into a 100mL three-necked round-bottomed flask, add 10.8g (5eq, 123.95mmol) morpholine, stir in an oil bath under magnetic force Heated to 120°C for reaction, followed by TLC until the reaction was complete, recovered excess morpholine under reduced pressure, added 25 mL of water, stirred for 1.0 h, then filtered with suction, washed the filter cake with water until the filtrate was colorless, and the yellow compound 2-(2-morpholine-5 -Nitrophenyl)ethanol (III), drying, yield 99.9%.
[0057] (2) Weigh 5.0g (19.82mmol) of the morpholine substitute (III) prepared above and add to a 100mL three-necked round-bottomed flask, 25mL of toluene is used as a solvent, and 2.16g of bis(trichloromethyl)carbonate is added dropwise at 100°C ( 0.36eq, 7.27mmol) of 5mL toluene solution, TLC traced the reaction until complete, an...
Embodiment 2
[0058] Example 2: Preparation of 4-(2-(2-(methylsulfoxide) ethyl)-4-nitrophenyl)morpholine (I)
[0059] (1) Add 5.0g (24.79mmol) 2-(2-chloro-5nitro)phenylethanol (II) into a 100mL three-necked round-bottomed flask, add 6.48g (3eq, 74.37mmol) morpholine, stir in an oil bath under magnetic force Heated to 120°C to react, followed by TLC to complete the reaction, recovered excess morpholine under reduced pressure, added 30mL of water, stirred for 1.0h, then filtered with suction, washed the filter cake with water until the filtrate was colorless, and the yellow compound 2-(2-morpholine-5 -Nitrophenyl)ethanol (III), drying, yield 99.9%.
[0060] (2) Weigh 5.0g (19.82mmol) of the above-prepared morpholine substitute (III) into a 100mL three-neck round-bottomed flask, use 20mL of chlorobenzene as a solvent, and add 1.94g of bis(trichloromethyl)carbonate dropwise at 100°C (0.33eq, 6.54mmol) of 5mL chlorobenzene solution, TLC tracked the reaction until complete, and recovered chlorob...
Embodiment 3-22
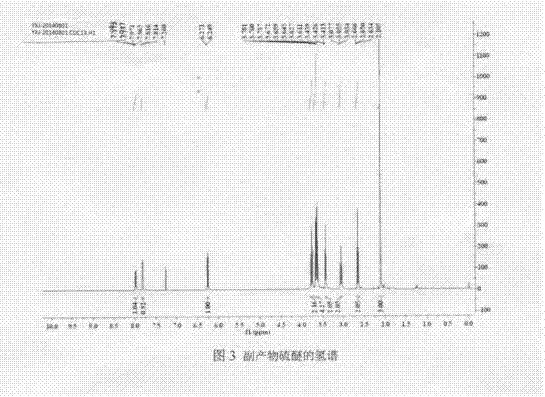
[0061] Embodiment 3-22 is the application of novel Swern reagent in the oxidation reaction of general alcohol and the intermediate 1-adamantanemethanol of saxagliptin drug for treating diabetes
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com