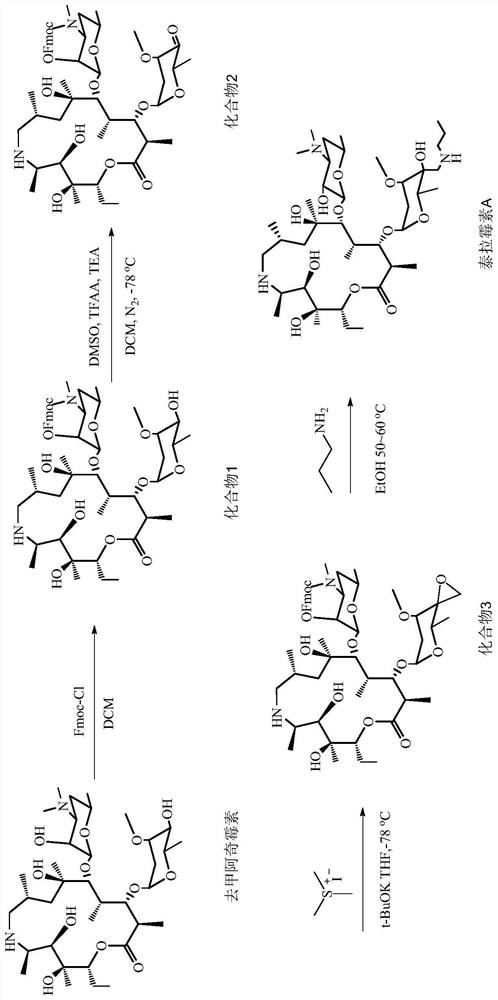
Tulathromycin synthesis method
A synthetic method and technology of telamycin, applied in chemical instruments and methods, organic chemistry, sugar derivatives, etc., can solve problems such as difficult removal of heavy metal residues, ring-opening isomerization of macrolides, unfavorable industrial production, etc. Achieve the effects of improving productivity, shortening reaction steps, and convenient process monitoring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: compound 1, i.e. the synthesis of 2'-Fmoc-nor azithromycin intermediate
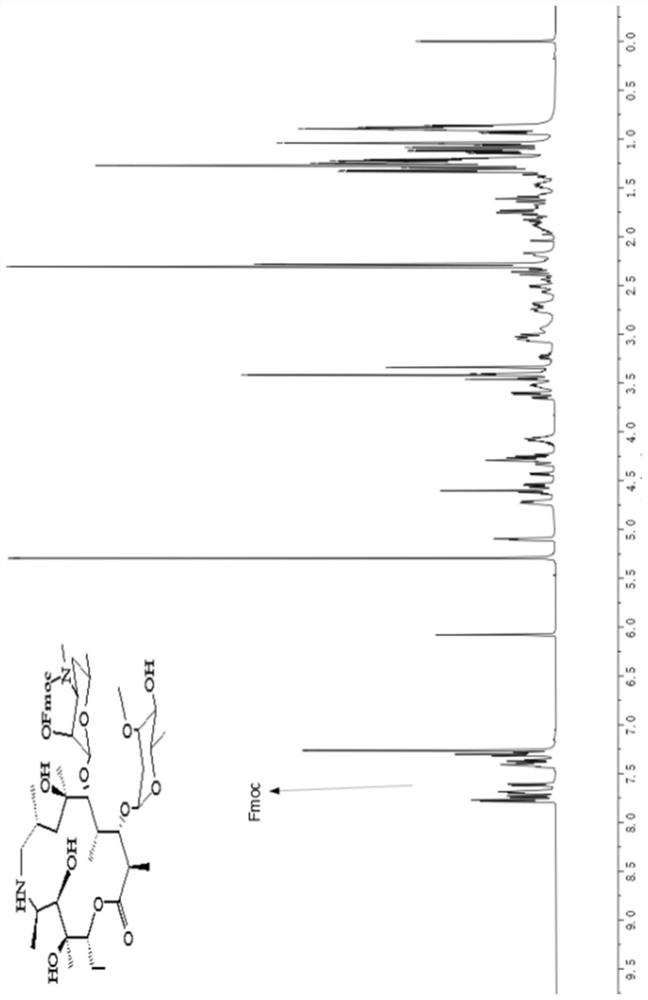
[0047] Add 100g (1eq) norazithromycin and 700mL dichloromethane into a 2L reaction flask, stir to dissolve, then add 29.4g sodium carbonate, stir for 30min, then slowly add Fmoc-Cl (43g, 1.2eq) dropwise to obtain a dichloromethane solution , After addition, stir at room temperature for 3h. TLC monitored the completion of the reaction. Add 200 mL of deionized water to the reaction solution, stir for 30 min, let stand to separate the liquid, dry (anhydrous sodium sulfate), filter, spin dry, and recrystallize the crude product with cyclohexane to obtain 113.2 g of white solid, yield 87%. H NMR spectrum see figure 2 .
Embodiment 2
[0051] Embodiment 2: compound 2, i.e. the synthesis of 2'-Fmoc-4 "-oxo-nor azithromycin intermediate
[0052] Add 100g (1eq) of 2'-Fmoc-norazithromycin intermediate, 43mL (5eq) of dimethyl sulfoxide and dichloromethane into a 3L reaction flask, cool to -78°C under nitrogen protection, and slowly add 168mL (10eq ) trifluoroacetic anhydride, keep warm at -78°C and stir for 1h, then slowly add 165mL (10eq) triethylamine dropwise, after the addition is complete, naturally raise the temperature to -20-30°C to continue the reaction for 1h, monitor the completion of the reaction by TLC, and add water to quench. Warm up to room temperature, add 700mL of water and 1200mL of dichloromethane, stir for 30min, separate the liquids, wash the organic phase with saturated brine and water once, dry over anhydrous sodium sulfate, filter, spin dry, and recrystallize the crude product with cyclohexane 85.8 g of white solid was obtained, with a yield of 86%.
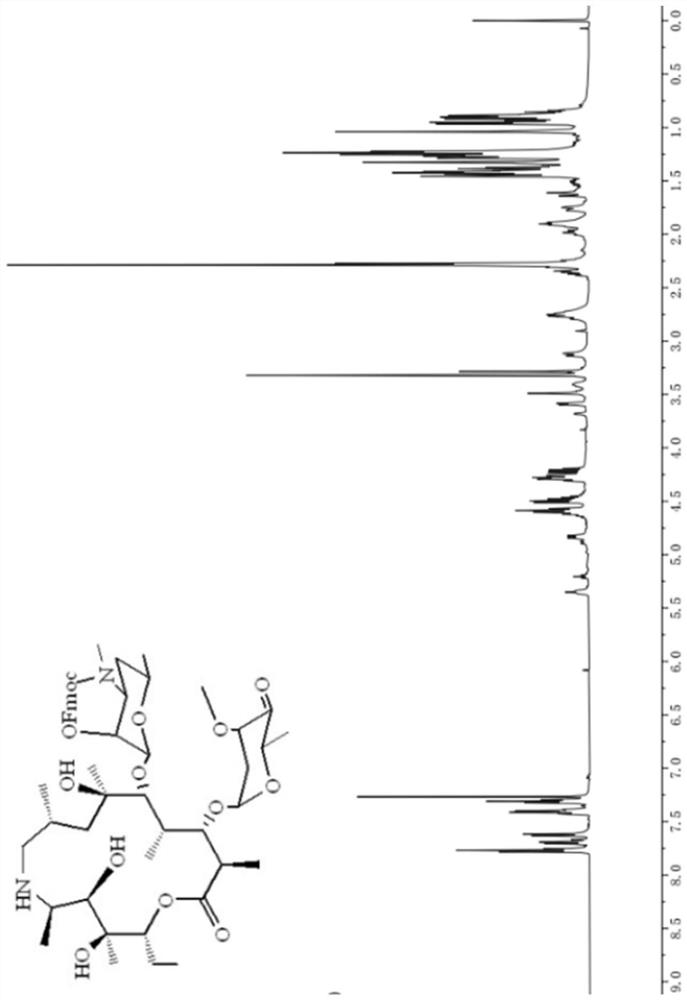
[0053] H NMR and C NMR spectra, see ...
Embodiment 3
[0054] Embodiment 3: compound 3, namely epoxy compound intermediate: the synthesis of 4 "-methylene epoxy-2'-Fmoc-nor azithromycin
[0055] Dissolve 36.9 (2eq) g of trimethylsulfur iodide in anhydrous tetrahydrofuran (100 mL) and cool to about -10°C, add 26.3 g (2 eq) of potassium tert-butoxide, and stir for 1 h to obtain sulfur ylide salt, which is set aside.
[0056] Dissolve 100g (1eq) of 2'-Fmoc-4"-oxonorazithromycin in anhydrous tetrahydrofuran (300mL), cool to -78°C, slowly add the above-prepared sulfur ylide dropwise under stirring, after the addition is complete, continue to keep warm Reaction at -78°C for 1h, TLC detection of the completion of the reaction. Add dropwise reaction saturated ammonium chloride solution to the reaction solution to extract, naturally rise to room temperature, dilute with water, extract with dichloromethane, separate liquids, combine organic phases, and wash with anhydrous sulfuric acid Dry over sodium, filter, and concentrate under reduced ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com