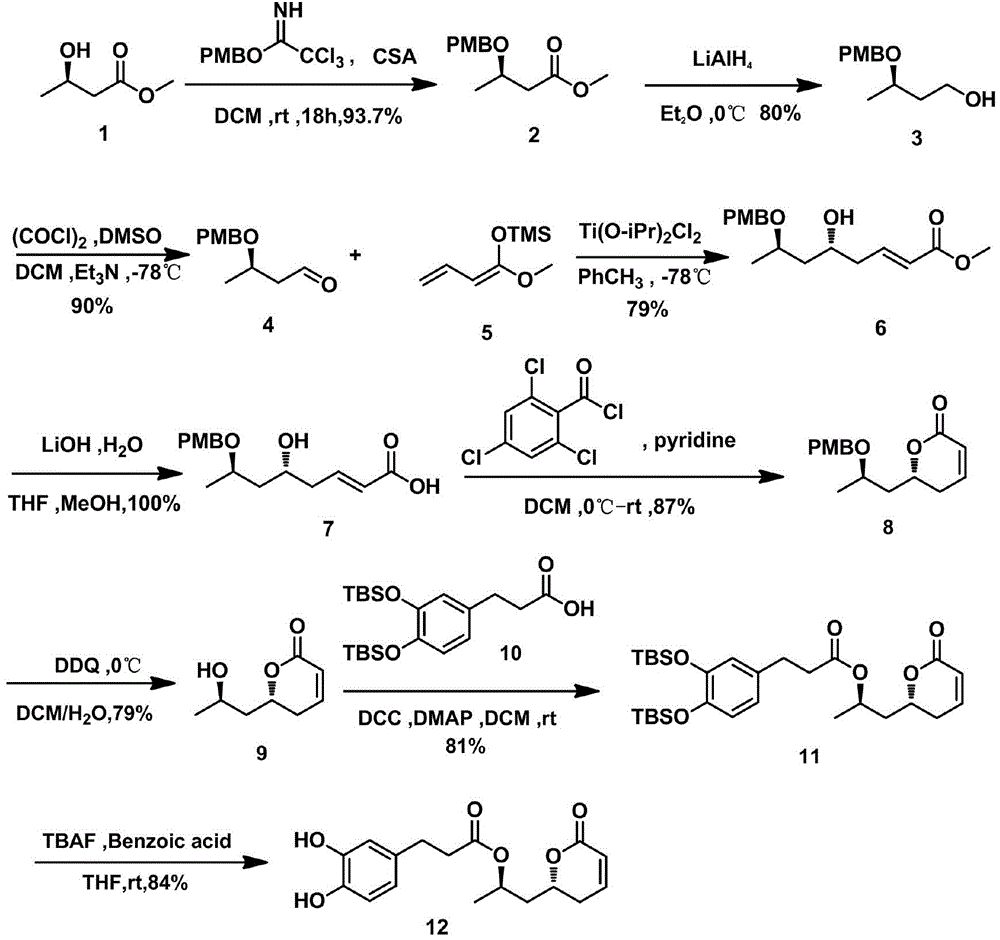
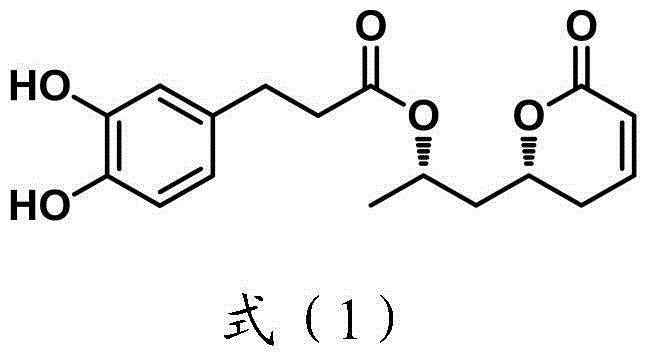
Method for synthesizing natural product Tarchonanthuslactone isomer
A technology of natural products and isomers, applied in the chemical industry, can solve the problems of slow rate, expensive reagents, and many side reactions, and achieve the effect of fast rate, easy operation, and few side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The synthesis of embodiment 1PMB ester
[0035] Under nitrogen protection, dissolve p-methoxybenzyl alcohol (5.565g) in ether (13ml), add (403mg, 60%) NaH at 0°C, stir at room temperature for 30min, then cool to 0°C, and add dropwise Trichloroacetonitrile (4.04ml), naturally warmed to room temperature and stirred at room temperature for 2h. After the reaction was completed, concentrate with a rotary evaporator, then add n-hexane (100ml) and rinse the precipitate, then filter with a small amount of diatomaceous earth, and concentrate the filtrate to obtain an orange oil, namely PMB ester (8g, 70.2%).
[0036] The synthesis of embodiment 2 formula 2 compounds
[0037] Under nitrogen, methyl trihydroxybutyrate (2.6ml) was first dissolved in dichloromethane (DCM, 56ml), and then PMB ester was dissolved in 56ml DCM. Under nitrogen protection, the diluted PMB ester solution was added dropwise to 1 solution, and then 535 mg of camphorsulfonic acid (CSA) was added, and stirre...
Embodiment 4
[0040] Synthesis (Swern oxidation) of embodiment 4 formula 4 compounds
[0041] Under the conditions of ensuring anhydrous and oxygen-free throughout the reaction process and using nitrogen protection, 1.75ml oxalyl chloride (COCl) 2 It was dissolved in 34ml of DCM, stirred at -60°C, and dimethyl sulfoxide (DMSO, 2.9ml) was added dropwise. After the mixture was stirred at low temperature for 15 min, the compound of formula 3 (2.85 g) was dissolved in DCM (34 ml) and added slowly to the reaction flask. After reacting for 1 h, triethylamine (Et 3 N, 13.22ml), after the addition of the material, the reaction mixture was taken out from the low temperature and placed in an ice-water bath, and then reacted for 2h. After the reaction, add saturated NaCl solution (90ml), extract with DCM (86ml×3), dry over anhydrous sodium sulfate, filter, concentrate and separate by column chromatography (ethyl acetate:petroleum ether=1:10), the The eluate was concentrated to give the compound of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com