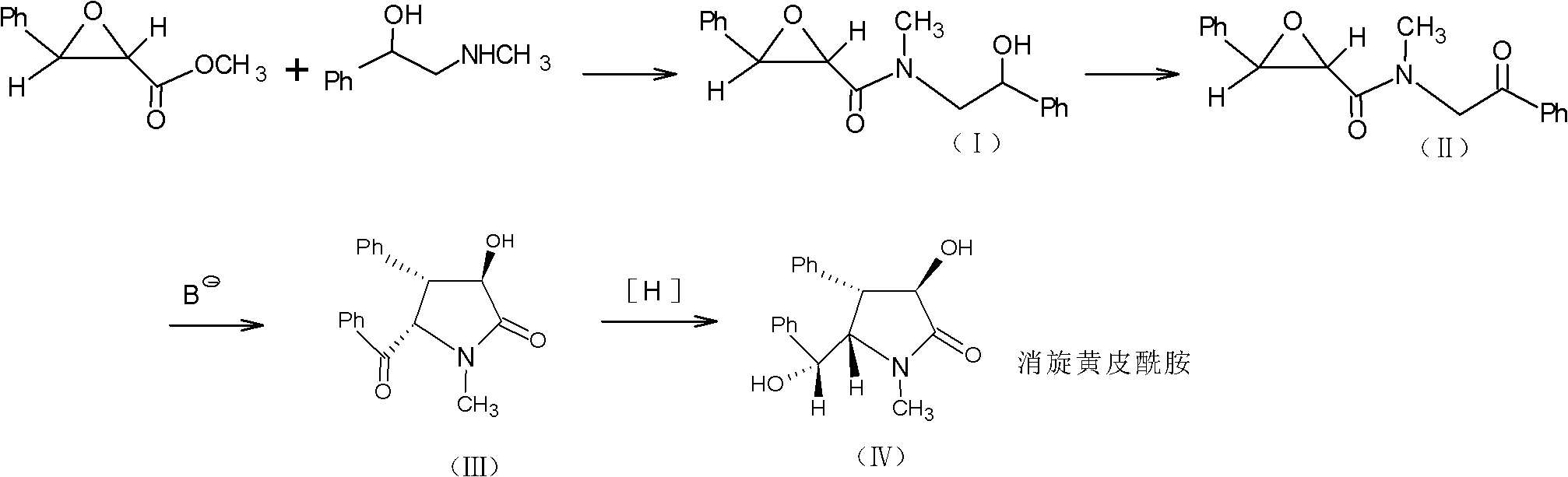
Method for preparing clausenamide intermediate by Swern oxidation process
A technology of phenyl glycidyl amide and compound, which is applied in the field of synthesis of N-methyl-N-phenacyl-3-phenyl glyceryl amide, can solve the problem of long filtration time, unsafe operation, Response is difficult to control and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 (contrast)
[0033] Preparation of N-methyl-N-phenacyl-3-phenyl glycidyl amide (KMnO 4 ·CuSO 4 Law)
[0034] Add 20g (0.067mol) of N-methyl-N-(2-hydroxy-2-phenyl)ethyl-3-phenylglycidylamide and 500ml of dichloromethane into a 1000ml single-necked bottle, heat slightly to completely dissolve the solid . After cooling to room temperature, add anhydrous CuSO 4 14.4g (0.09mol) and KMnO 4 42.4g (0.266mol, above 200 mesh). After stirring for 2 hours, the reaction was complete by TLC. Filter with diatomaceous earth, and wash with 500ml of dichloromethane several times until the washing solution shows only weak product spots on TLC. The filtrate and washings were combined, concentrated under reduced pressure to dryness to obtain about 12 g of light yellow oil. Treat the oily substance with 100ml of ether, stir until the oily substance is completely broken, and a white solid precipitates out. Cool to +3°C, collect the solid by filtration, and wash with cold...
Embodiment 2
[0035] Embodiment 2 (contrast)
[0036] Large-scale preparation of N-methyl-N-phenacyl-3-phenylglyceramide (KMnO 4 ·CuSO 4 Law)
[0037] Put 20kg of compound (I) into a 1000L reactor, suck in 670kg of dichloromethane, heat slightly to dissolve the solid completely, add 14.4kg of anhydrous copper sulfate and 42.4kg of potassium permanganate after cooling to room temperature. Stir the reaction at room temperature. Spot the plate after 10 hours to detect that the reaction is complete, centrifuge, and rinse the filter cake with dichloromethane repeatedly until the filtrate TLC detects that the fluorescence of the product is weak. The filtrates were combined and concentrated to dryness under reduced pressure to obtain 10 kg of yellow oil.
[0038] Stir the oily product with 50 L of diethyl ether to precipitate a solid under freezing conditions, put it in a freezer overnight, filter it with suction, and wash the solid with frozen diethyl ether to obtain 8.5 kg of a white solid, w...
Embodiment 3
[0040]Preparation of N-methyl-N-phenacylmethyl-3-phenylglyceramide (PCC method)
[0041] 29g PCC(CrO 3 PyHCl, 0.13mol) was dissolved in dichloromethane, and it was added dropwise to 20g (0.067mol) N-methyl-N-(2-hydroxyl-2-phenyl)ethyl-3-phenylglyceramide at room temperature (1) in the 500ml dichloromethane solution, reacted at room temperature for 2 hours, TLC showed that the reaction was complete, suction filtered, and the filter cake was washed with dichloromethane. The filtrates were combined, the organic phase was washed with saturated brine, dried, filtered with suction, and concentrated to dryness under reduced pressure. It was separated with silica gel and eluted with ethyl acetate:petroleum ether (3:2). The eluate containing the product was collected and concentrated to obtain 10.5 g of the product. The yield of the crude product was 58.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com