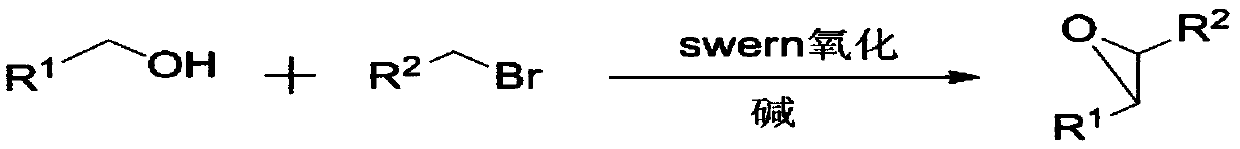Method used for direct synthesis of epoxy compounds from alcohol
An epoxy compound and synthesis method technology, applied in the directions of organic chemistry, bulk chemical production, etc., can solve the problems of difficult treatment process, harsh operating conditions, large three-waste discharge, etc., and achieves cheap and easy-to-obtain reaction reagents and easy preparation process. Controls, substrates for a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
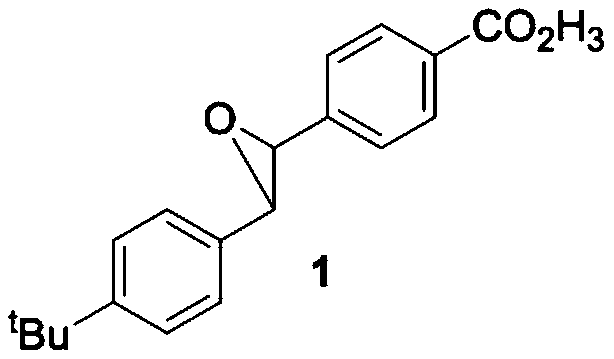
[0036] Embodiment 1 A kind of method of directly synthesizing epoxy compound 1 by alcohol
[0037] (11) Dissolve 1.1 mmol of oxalyl chloride in 1 mL of anhydrous dichloromethane at -78°C in a nitrogen atmosphere, slowly add 2.2 mmol of anhydrous dimethyl sulfoxide dropwise to the reaction system, and stir for 30 minutes to obtain A1;
[0038] (12) Dissolve 1.1 mmol of 4-(hydroxymethyl) methyl benzoate in 0.5 mL of anhydrous dichloromethane, slowly add it dropwise to A1, stir for 2.5 h, and slowly add 5.5 mmol of triethylamine to the reaction system, Stir for 1h to obtain B1;
[0039] (13) TLC (thin layer chromatography) detection, the raw materials disappeared, the reaction was moved to room temperature, and 1.1mmol 4-tert-butylbenzyl bromide, 5.5mmol lithium hydroxide, 0.2mL water and 0.5mL were added to B1 at room temperature Dimethyl sulfoxide, reacted at 20°C for 12 hours, detected by TLC (thin layer chromatography), the raw material point disappeared, and the reaction wa...
Embodiment 2
[0044] Embodiment 2 A kind of method of directly synthesizing epoxy compound 2 by alcohol
[0045] (21) Dissolve 1.1 mmol of acetic anhydride in 1 mL of anhydrous dichloromethane at -70°C in a nitrogen atmosphere, slowly add 5.5 mmol of anhydrous dimethyl sulfoxide dropwise to the reaction system, and stir for 50 min to obtain A2;
[0046] (22) Dissolve 1.1mmol of 4-cyanobenzyl alcohol in 0.5mL of anhydrous dichloromethane, slowly add it dropwise to A2, stir for 1h, slowly add 8.8mmol of triethylamine to the reaction system, stir for 1h, and obtain B2 ;
[0047] (23) TLC (thin-layer chromatography) detection, the raw materials disappeared, the reaction was moved to room temperature, and 1.1mmol 4-tert-butylbenzyl bromide, 7.7mmol cesium carbonate, 0.2mL water and 0.5mL tert-butylbenzyl bromide were added to B2 at room temperature Butanol, reacted at 35°C for 15h, detected by TLC (thin layer chromatography), the raw material point disappeared, and the reaction was stopped to o...
Embodiment 3
[0052] Embodiment 3 A kind of method of directly synthesizing epoxy compound 3 by alcohol
[0053] (31) Dissolve 2.2 mmol of acetic anhydride in 1 mL of anhydrous dichloromethane at -60°C in a nitrogen atmosphere, slowly add 3.3 mmol of anhydrous dimethyl sulfoxide dropwise to the reaction system, and stir for 10 min to obtain A3;
[0054] (32) Dissolve 1.1mmol of 4-tert-butylbenzyl alcohol in 0.5mL of anhydrous dichloromethane, slowly add it dropwise to A3, stir for 1.5h, slowly add 3.3mmol of DBU to the reaction system, stir for 2.5h to obtain B3 ;
[0055] (33) TLC (thin layer chromatography) detects that the raw material disappears, and the reaction is moved to room temperature, and 1.1 mmol 4-methyl-2,3,5,6-tetrafluoro-benzyl bromide is added to B3 at room temperature, 8.8 Mmol sodium carbonate, 0.2mL water and 0.5mL isopropanol were reacted at 35°C for 20h, detected by TLC (thin layer chromatography), the starting point disappeared, and the reaction was stopped to obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com