Patents
Literature
73 results about "Phenyl alcohol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phenyl Ethyl Alcohol Uses Phenyl Ethyl Alcohol is used for the treatment, control, prevention, & improvement of the following diseases, conditions and symptoms: The following is a list of possible side-effects that may occur in medicines that contain Phenyl Ethyl Alcohol. This is not a comprehensive list.
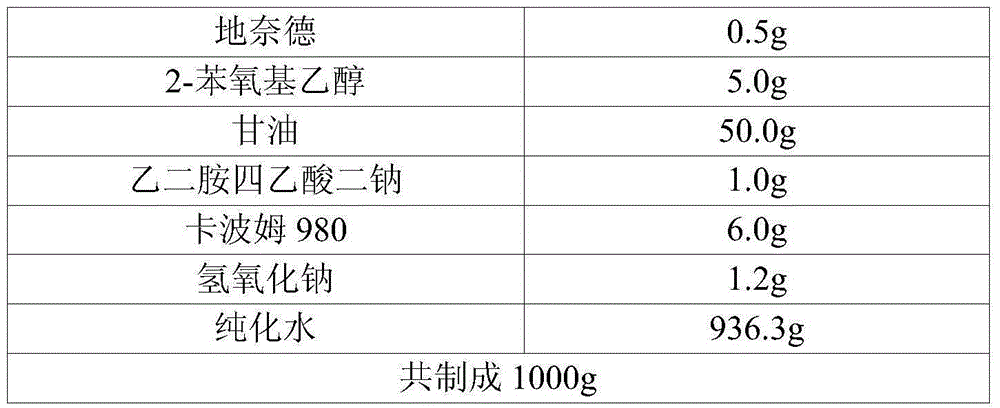
Preserved pharmaceutical formulations
InactiveUS7109161B1Reduce concentrationImprove compatibilityAntibacterial agentsBiocideMedicinePhenethyl alcohol
The present invention is directed to the use of benzethonium chloride, alone or in combination with phenoxyethanol or phenyl ethyl alcohol, to provide anti-microbial activity in pharmaceutical compositions. The present invention also provides methods of using benzethonium chloride, alone or in combination with phenoxyethanol or phenyl ethyl alcohol, to inhibit microbial growth in pharmaceutical compositions.
Owner:AVENTISUB LLC
Compositions containing phenethyl aryl esters as solubilizing agents for active organic compounds
ActiveUS20050008586A1Low color requirementReduce the smellBiocideCosmetic preparationsArylSunscreen agents
An active or functional organic compound is solubilized in a phenylethyl ester, e.g. an aryl carboxylic ester of 2-phenylethyl alcohol, as a solvent, cosolvent or additive, to form a composition thereof. Representative active or functional organic compounds include personal care products, e.g. sunscreens containing UVA / UVB absorbing compounds, such as avobenzone and benzophenone-3. Such compositions also show increased critical wavelength and UVA / UVB absorbance ratio performance properties.
Owner:ISP INVESTMENTS LLC
Compositions containing phenethyl aryl esters as solubilizing agents for active organic compounds
ActiveUS20050019280A1Improve performanceRaise the ratioCosmetic preparationsBiocideSunscreen agentsPhenethyl alcohol
Owner:ISP INVESTMENTS LLC
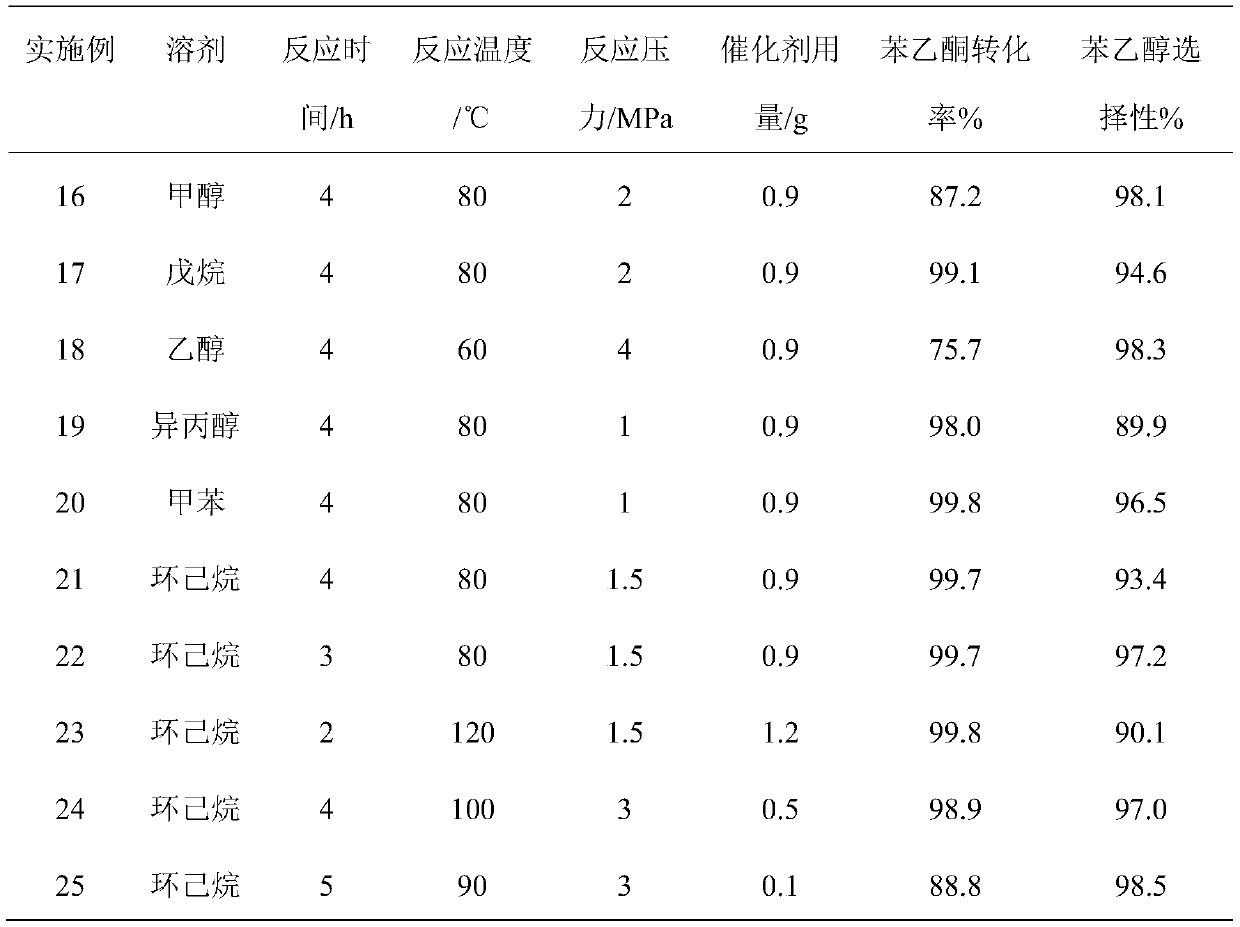
Method for separating acetophenone and alpha-phenethyl alcohol from mixture of acetophenone and alpha-phenethyl alcohol
ActiveCN104447267APrevent oxidationKeep natural propertiesOrganic compound preparationHydroxy compound preparationDistillationPhenethyl alcohol
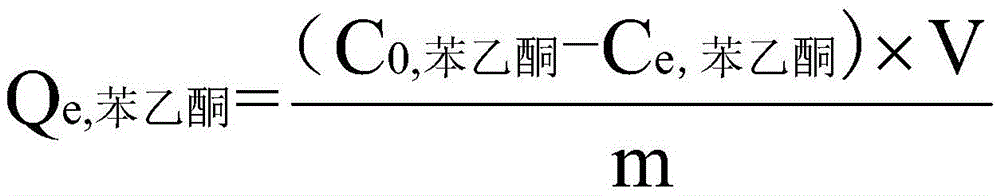
The invention discloses a method for separating acetophenone and alpha-phenethyl alcohol from a mixture of the acetophenone and the alpha-phenethyl alcohol. The method comprises the following steps: (1) adding acetophenone and alpha-phenethyl alcohol to a material tank, controlling the temperature, the evaporation temperature, the reflux ratio and the vacuum degree of a molecular distillation and rectification coupling system rectification column, and respectively separating to obtain two components of a light component and a heavy component; (2) adding the light component to the material tank, controlling the temperature, the evaporation temperature, the reflux ratio and the vacuum degree of the molecular distillation and rectification coupling system rectification column, so as to obtain high-purity acetophenone; and (3) adding the heavy component to the material tank, controlling the temperature, the evaporation temperature, the reflux ratio and the vacuum degree of the molecular distillation and rectification coupling system rectification column, so as to obtain high-purity alpha-phenethyl alcohol. According to the separation method, the defects of conventional reduced pressure rectification and molecular distillation are compensated; the method is short in separation time and simple in flow; low-energy consumption and high-efficiency separation of two mixtures can be achieved; and the method is economic, environment-friendly, and easy to achieve industrialization.
Owner:HANDWAY TECH FOSHAN +1
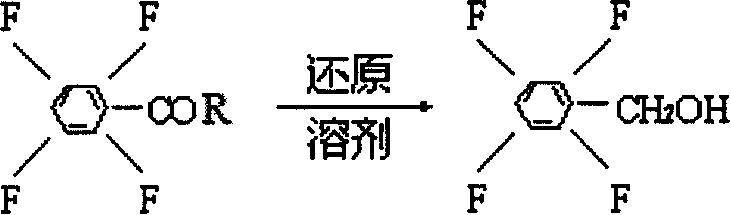
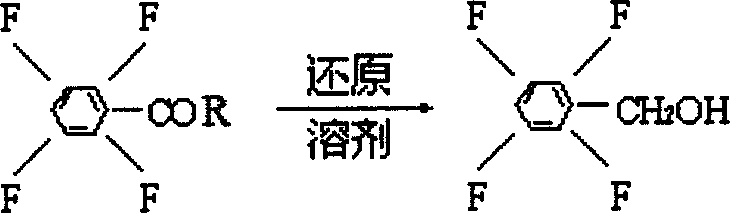
Process preparing 2,3,5,6-phyenyl methanol tetrafluoride
InactiveCN1900037AOrganic compound preparationHydroxy compound preparationBenzoyl chlorideTransfluthrin
The present invention is process of preparing 2, 3, 5, 6-tetrafluoro phenyl alcohol as one important material for synthesizing tetrafluoro phenyl pyrethrin through the reduction reaction of 2, 3, 5, 6-tetrafluoro benzoyl chloride material under the action of solvent, reductant and assistant.
Owner:常州康美化工有限公司
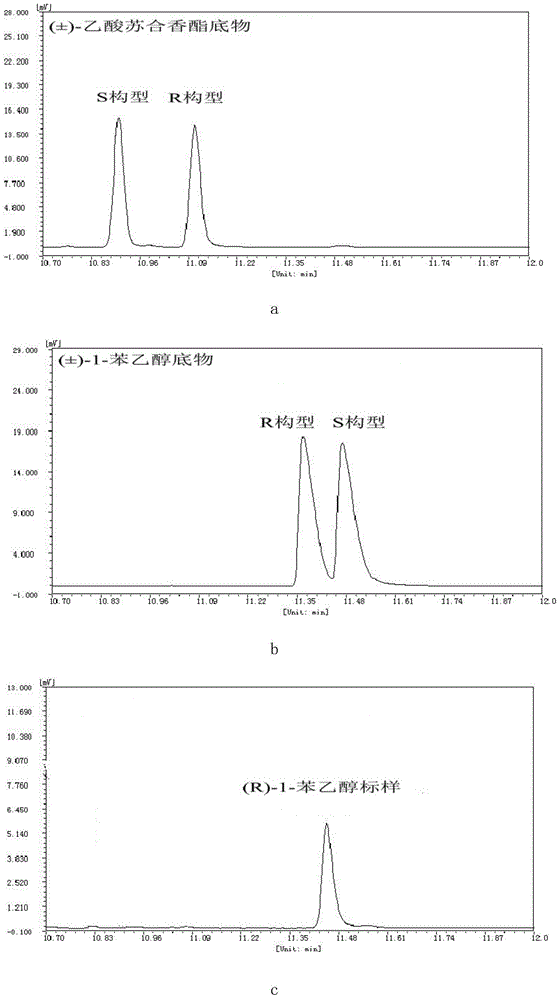
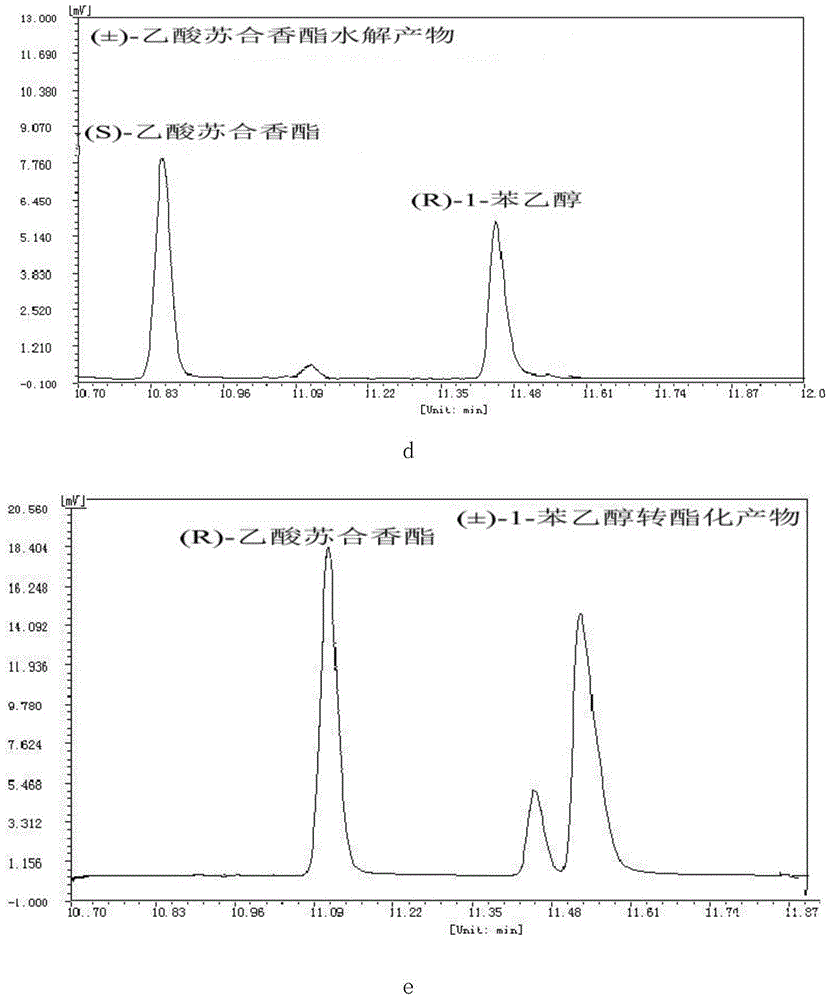
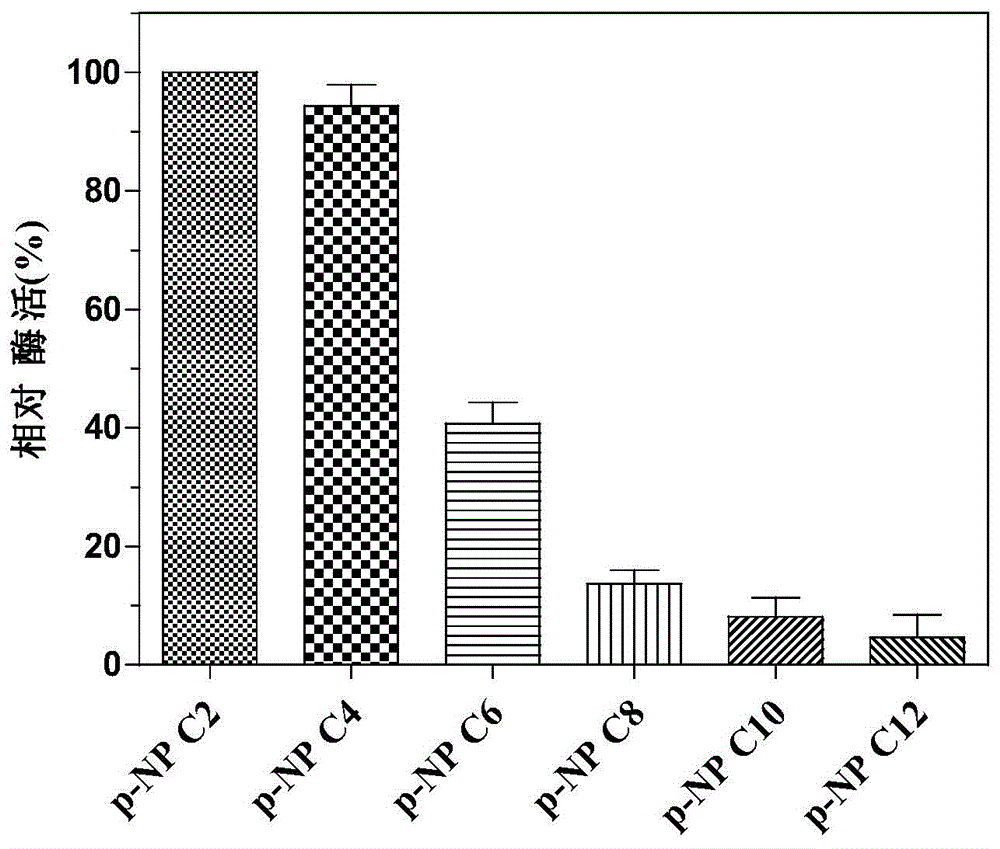
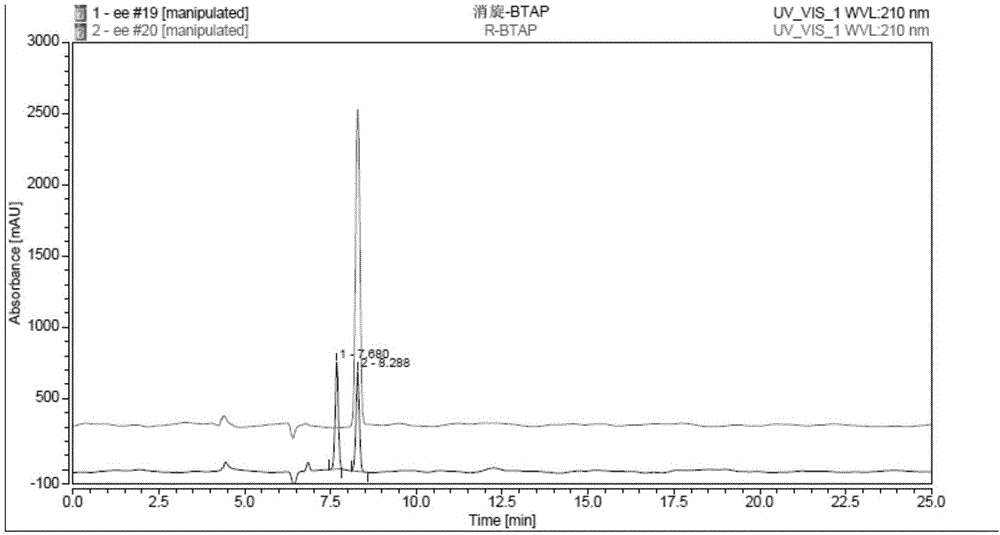
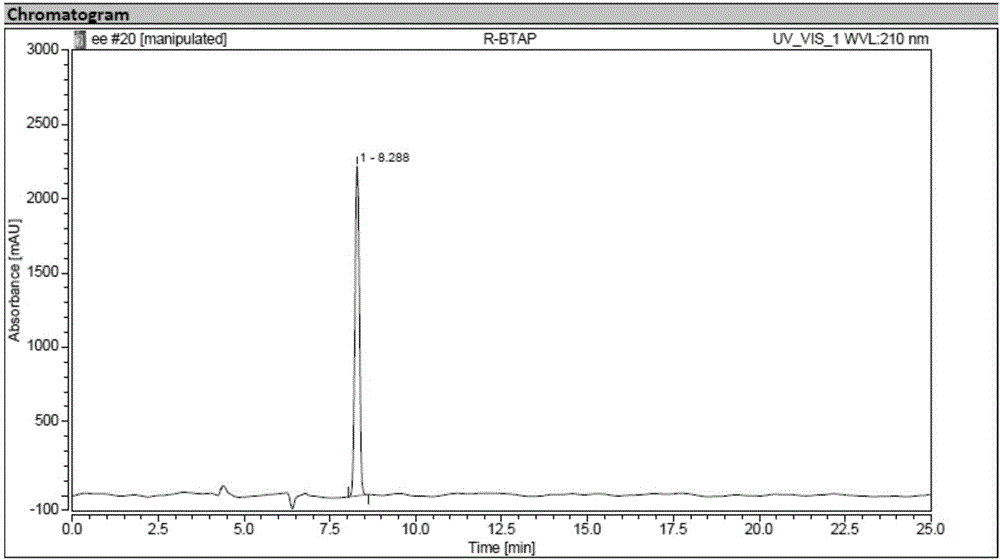
Novel esterase, encoding gene and application thereof in splitting (+/-)-1-phenethyl alcohol and (+/-)-styralyl acetate
ActiveCN104962533AMild reaction conditionsNo pollution in the processHydrolasesFermentationAcetic acidEnantiomer
The invention discloses novel esterase, encoding gene and an application thereof in splitting (+ / -)-1-phenethyl alcohol and (+ / -)-styralyl acetate. The amino acid sequence of the esterase is shown in SEQ ID NO.2 and the nucleotide sequence is shown in SEQ ID NO.1. Asymmetric hydrolysis is carried out on the (+ / -)-styralyl acetate in an aqueous phase and (S)-styralyl acetate with the enantiomer excess value being 95% and (R)-1-phenethyl alcohol with the enantiomer excess value being more than 99% are obtained; and asymmetric transesterification is carried out on the (+ / -)-1-phenethyl alcohol in an organic phase and (R)-styralyl acetate with the enantiomer excess value being 99% and (S)-1-phenethyl alcohol with the enantiomer excess value being more than 70% are obtained. Compared with the traditional chemical splitting phase, the novel esterase, the encoding gene and the application disclosed by the invention have the advantages of mild reaction condition, no pollution and high enantiomer excess value and the obtained product can be used for synthesizing corresponding medicines after being simply purified.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
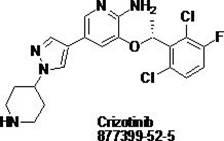
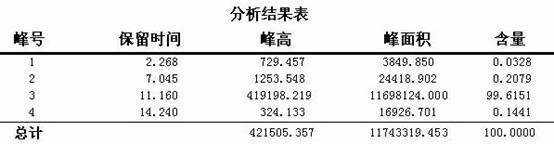
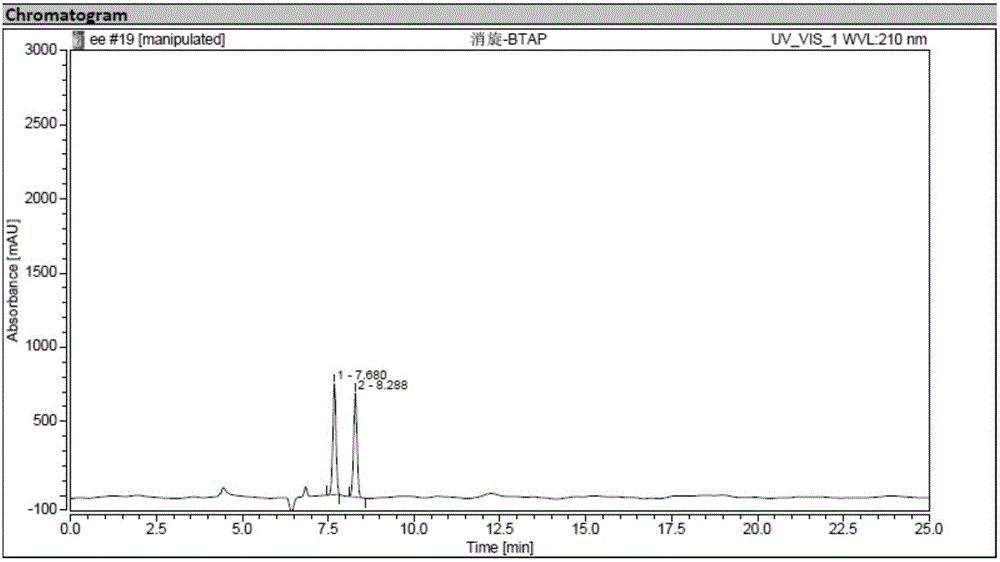
Synthesis method of crizotinib serving as antitumor molecular targeting medicament
ActiveCN102532106AReduce processing stepsEmission reductionOrganic chemistryAntineoplastic agentsPtru catalystEthyl group
The invention discloses a synthesis method of crizotinib serving as an antitumor molecular targeting medicament, which belongs to the field of pharmacy and relates to a novel splitting process of a chiral isomer of a crizotinib precursor and a synthesis method of an intermediate. (S)-1-(2,6-dichloro-3-fluorophenyl)ethanol is prepared by splitting a 1-(2,6- dichloro-3-fluorophenyl)ethanol racemic body into S-alcohol and R-alcohol with a catalytic splitting method for combining Boc-L-proline(N-tert-butoxycarbonyl-L-proline), paratoluenesulfonic acid serving as a catalyst and 1-(3-dimethylaminopropyl)-3-ethyl carbodiimide hydrochloride, separating and purifying; the yield is 60 percent; and the excessive fraction ee) of the chiral enantiomer is 99 percent. According to the method, time is shortened, cost is reduced, the generation of waste acids is avoided, environmental pollution is lowered, column chromatography isolation is not required, and industrial production is easier to implement.
Owner:JINAN TRIO PHARMATECH
Bio-preparation method for (R)-3,5-bis(trifluoromethyl) phenyl ethanol
The invention provides a bio-preparation method for (R)-3,5-bis(trifluoromethyl) phenyl ethanol (I). The method specifically comprises the following steps: (a) an asymmetric reduction reaction is performed in a liquid reaction system with a compound shown in the formula II as a substrate in presence of a coenzyme under catalysis of carbonyl reductase, and a compound shown in the formula I is prepared, wherein in the reaction system, the concentration of the compound shown in the formula II is 50-1,000 g / L; (b) the compound shown in the formula I is optionally separated from the reaction system subjected to the reaction in the step (a). The invention further provides the reaction system. The reaction system comprises (i) a waterborne solution, (ii) the substrate which is the compound shown in the formula II, (iii) the coenzyme, (iv) carbonyl reductase, (v) a co-substrate and (vi) an enzyme for regenerating the coenzyme.
Owner:SHANGHAI INST OF PHARMA IND +1
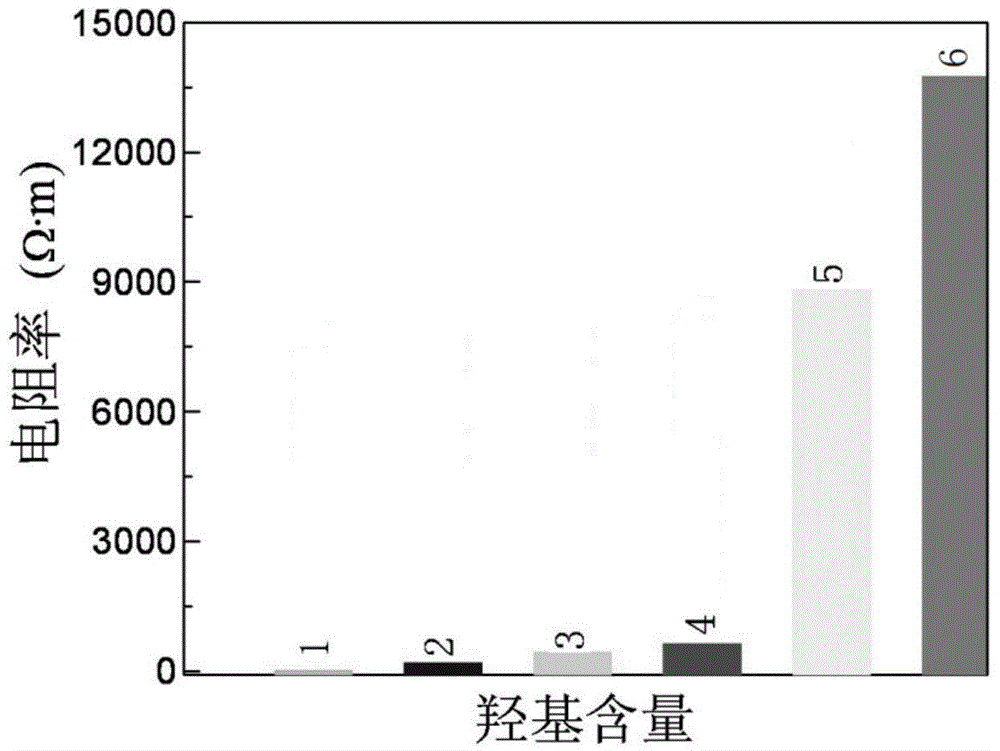
Preparation method of hydroxylated graphene powder with controllable conductive performance
The invention discloses a preparation method of hydroxylated graphene powder with a controllable conductive performance. The electrical resistivity of the powder is controlled in a range of 10<-4>ohm.m-10<5>ohm.m. The method comprises the following steps: ultrasonically treating oxidized graphene in distilled water to obtain a uniformly dispersed oxidized graphene aqueous solution; adding hydrazine hydrate and ammonium water, and condensing and refluxing in an oil bath to obtain a turbid liquid of graphene and water; then, adding amino phenyl alcohol and isoamyl nitrite and condensing and refluxing to obtain a hydroxylated graphene aqueous solution; filtering to obtain a neutral solution; and freezing and drying to obtain the hydroxylated graphene powder with the controllable conductive performance. The hydroxylation degree of graphene is controlled by controlling the rate of charge of amino phenyl alcohol and oxidized graphene so as to control the conductivity of the hydroxylated graphene powder. The hydroxylated graphene powder is safe, environmental friendly, is free of inert gas protection and foreign ions, is fluffy and is not agglomerated, is high in degree of purity, is excellent in quality and is good in solubleness.
Owner:AVIC BEIJING INST OF AERONAUTICAL MATERIALS
S-(-)-1-{4-[2-(allyloxy)-ethyl]phenoxy}-3-isopropylamino propan-2-ol, process for preparation thereof and process for preparation of S-(-)betaxolo
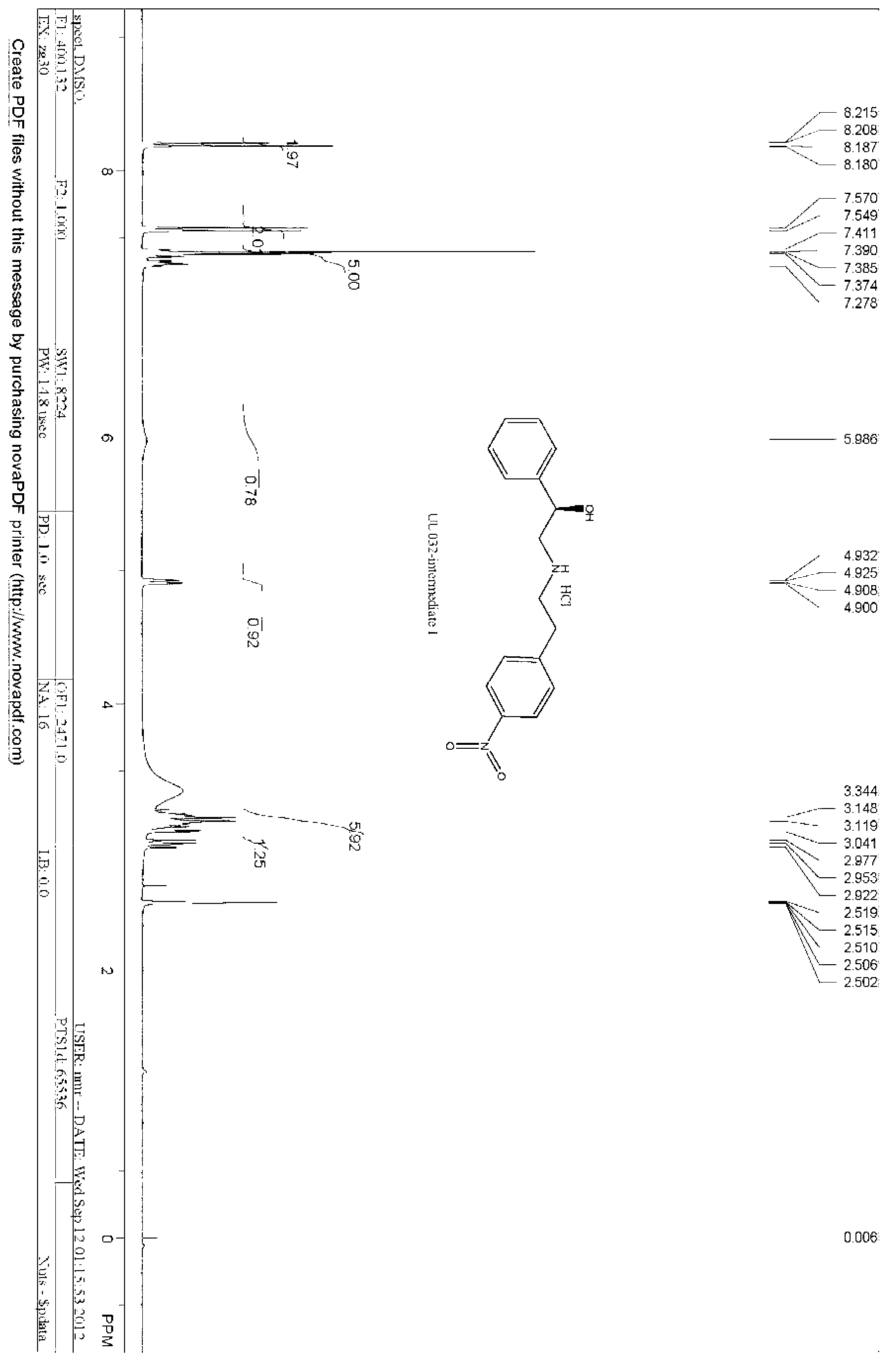
InactiveUS6989465B1Reduce usagePrevent steppingOrganic compound preparationOptically-active compound separationAlcoholMedicinal chemistry
The present invention relates to a novel compound S-(−)-1-{4-[2-(allyloxy)-ethyl] phenoxy}-3-isopropylamino propan-2-ol of formula 1 and to a process for the preparation thereof. More particularly the present invention relates to a process for preparing S-(−)-1-{4-[2-(allyloxy)-ethyl]phenoxy}-3-isopropylamino propan-2-ol of formula 1 by selective allylation of p-hydroxy phenyl ethanol. The present invention also relates to a process for conversion thereof to S-(−)-betaxolol of formula 2
Owner:COUNCIL OF SCI & IND RES
Preparation and application of novel Swern reagent
ActiveCN105585540AInhibition releaseRaise the reaction temperatureOrganic compound preparationCarbonyl group formation/introductionMorpholineReaction temperature
The invention discloses 4-(2-(2-methyl sulfoxide)ethyl)-4-nitrobenzene)morpholine shown in the formula (I) and preparation and application thereof. A preparation method includes the steps that 2-(2-chlorine-5 nitro)phenethyl alcohol shown in the formula (II) and morpholine are mixed to prepare 2-(2-morpholine-5-nitrobenzene)ethanol shown in the formula (III); bis(trichloromethyl)carbonate ester, a sodium methyl mercaptide aqueous solution and an aqueous hydrogen peroxide solution are sequentially added dropwise to 2-(2-morpholine-5-nitrobenzene)ethanol shown in the formula (III), and finally 4-(2-methyl sulfoxide)ethyl)-4-nitrobenzene)morpholine is prepared. According to the application of 4-(2-methyl sulfoxide)ethyl)-4-nitrobenzene)morpholine, the obtained Swern reagent reacts with an alcohol compound shown in the formula (IV), and aldehyde or ketone is prepared after after-treatment. The defects of an existing Swern oxidation method are overcome, generation of a stink byproduct dimethyl sulfide and toxic carbon monoxide is avoided from the source, the reaction temperature is increased to be -30 DEG C to 0 DEG C, and an odorless byproduct novel sulfur ether can be recycled and reused. The formulas are shown in the description.
Owner:ZHEJIANG UNIV OF TECH
Anti-pilling agent for wool fabric and preparation method of anti-pilling agent
InactiveCN104195817AImprove pilling resistanceHydrophilicity unchangedFibre treatmentPolymer scienceSodium phosphates
The invention provides an anti-pilling agent for a wool fabric. The anti-pilling agent consists of the following materials in parts by mass: 10-20 parts of ethyl acrylate, 10-20 parts of polyoxyethylene octyl phenyl alcohol, 10-20 parts of zinc nitrate, 10-15 parts of paraffin, 5-8 parts of stearic acid, 5-8 parts of sodium sulfate, 3-6 parts of ammonium thioglycolate, 2-5 parts of potassium tartrate, 1-3 parts of chitosan, 1-2 parts of sodium hexametaphosphate, 80-90 parts of ethyl alcohol and 300-400 parts of water. By virtue of the anti-pilling agent, the anti-pilling grade of the wool fabric can be enhanced by over two levels and the hydrophilicity can be kept the same fundamentally.
Owner:广东新龙新服饰有限公司
Process for preparation of 2-phenyl ethanol
InactiveUS20050131258A1Reduce usageEasy to separateOrganic compound preparationOxygen compounds preparation by reductionStyrene oxideSilicon dioxide
The present invention provides an improved process for preparation of 2-phenyl ethanol. More specifically, the present invention relates to a process for preparing 2-phenyl ethanol by catalytic transfer hydrogenation of styrene oxide, in the presence of a supported transition metal catalyst. The catalyst system comprises of a palladium supported on silica, alumina, clay or charcoal.
Owner:COUNCIL OF SCI & IND RES
Synthesis method of (R)-2-p-nitrobenzene ethylamine-1-phenethyl alcohol and salt thereof
InactiveCN103193658AReduce pollutionSuitable for industrialized mass productionOrganic compound preparationAmino-hyroxy compound preparationSynthesis methodsPhenethyl alcohol
The invention provides a synthesis method of (R)-2-p-nitrobenzene ethylamine-1-phenethyl alcohol and salt thereof, belongs to the technical field of medicine synthesis and solves the problems that in the prior art the cost is high, the product yield is low, the synthesis method is not applicable to large-scale industrial production, and the like. The synthesis method comprises the following steps of: under the action of an oxidant, oxidizing hydroxyl on p-nitrobenzene ethanol into an aldehyde group so as to obtain p-nitrobenzene acetaldehyde; under the action of a reducing agent, carrying out dehydration, condensation and reduction on amino on (R)-2-amino-1-phenethyl alcohol and the aldehyde group on p-nitrobenzene acetaldehyde so as to obtain (R)-2-p-nitrobenzene ethylamine-1-phenethyl alcohol; and mixing a rough product of (R)-2-p-nitrobenzene ethylamine-1-phenethyl alcohol with an acid so as to generate precipitate or stirring till solid (R)-2-p-nitrobenzene ethylamine-1-phenethyl alcohol salt is separated out. The synthesis method of (R)-2-p-nitrobenzene ethylamine-1-phenethyl alcohol and the salt thereof is low cost and high in product yield, and is applicable to large-scale industrial production.
Owner:SUZHOU UUGENE BIOPHARMA
Method for preparing alpha-phenethyl alcohol by catalyzing acetophenone hydrogenation with supported amorphous alloy
InactiveCN110368946ASave raw materialsThe process is simple and safe and stableOrganic compound preparationHydroxy compound preparationPhenethyl alcoholAcetophenone
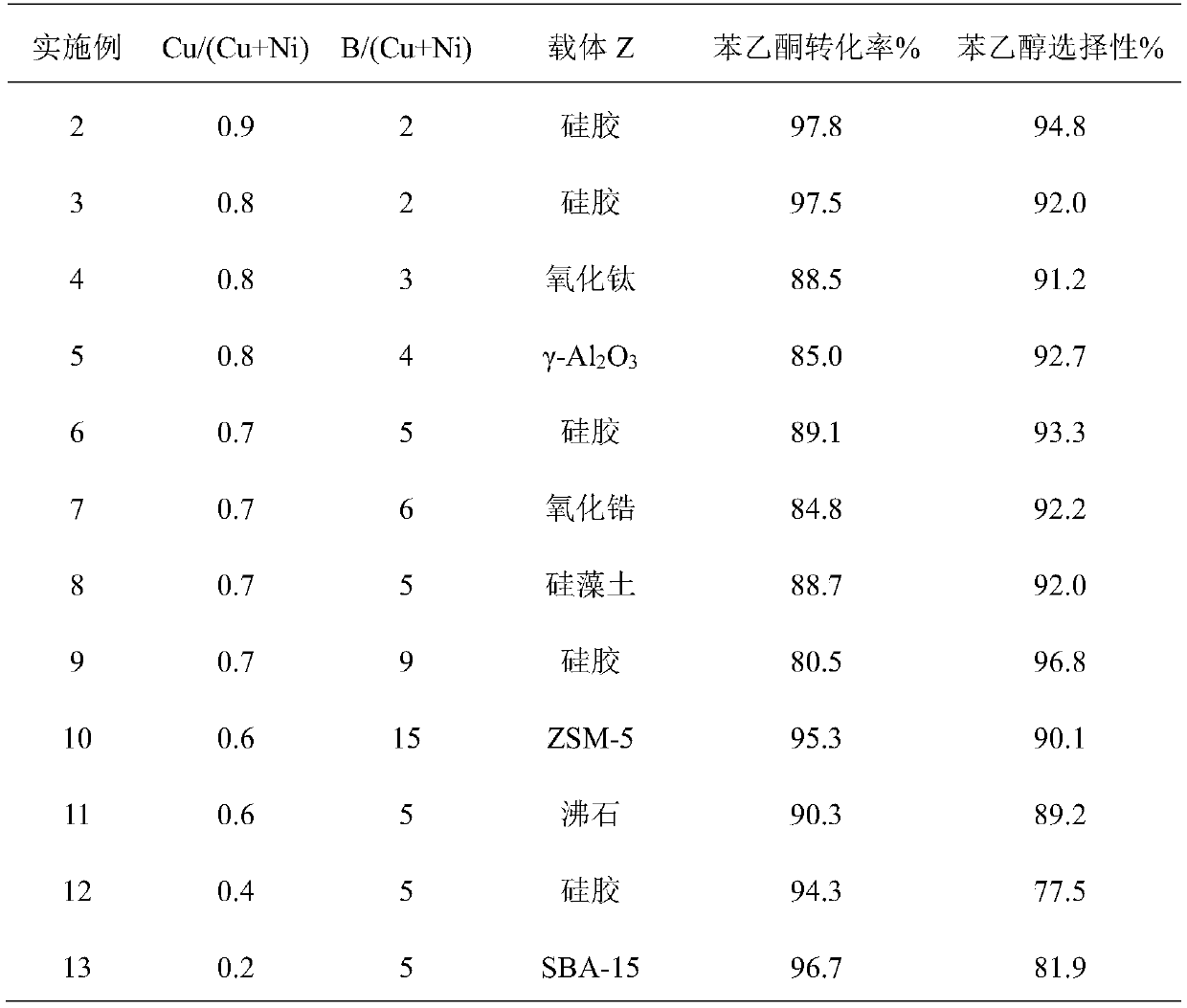
The invention belongs to the technical field of chemical engineering and in particular relates to a method for preparing alpha-phenethyl alcohol by catalyzing acetophenone hydrogenation with a supported amorphous alloy. An amorphous alloy catalyst consists of copper, nickel, boron and a porous carrier material Z, wherein the copper mainly exists in a mode of a Cu-Ni-B amorphous alloy. The preparation method comprises the following step: reducing active components, namely copper and nickel, into a carrier through BH4-. The catalyst is low in raw material price and safe to use, is applied to reactions for preparing phenethyl alcohol through acetophenone hydrogenation, and has good stability and selectivity when being compared with a conventional nickel-based catalyst; and when the acetophenone conversion rate is up to 98% or greater, the selectivity of alpha-phenethyl is up to 97%, and the activity of the catalyst after 300 hours of reactions is still maintained at a high level.
Owner:QINGDAO UNIV OF SCI & TECH
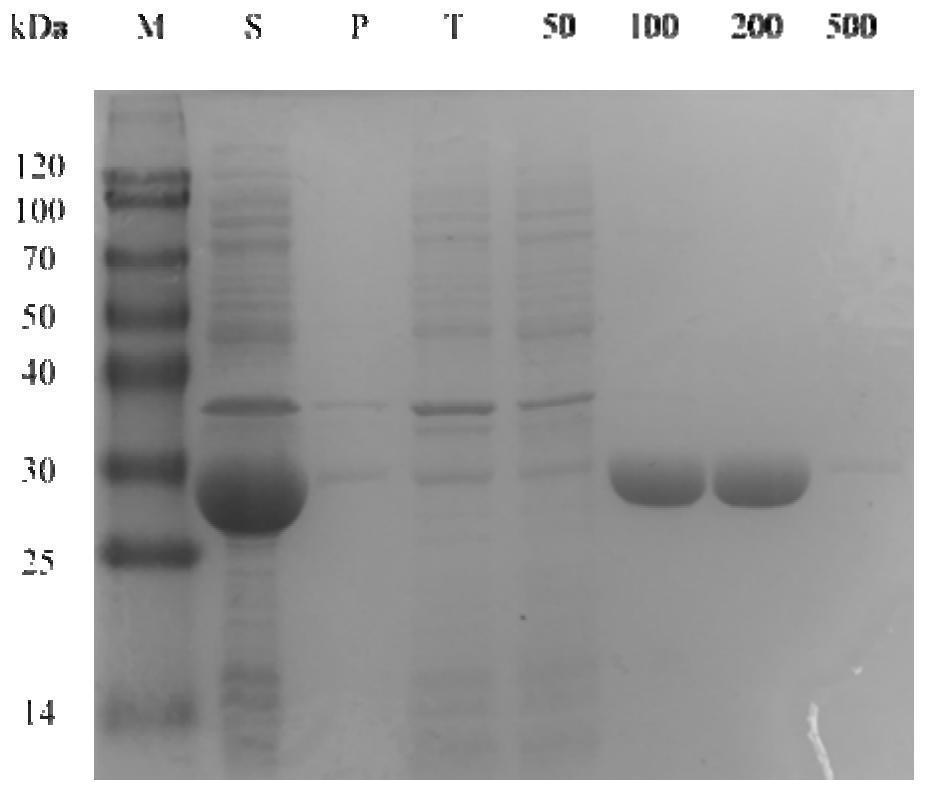
Alcohol dehydrogenase mutant with improved activity and stereoselectivity, recombinant vector of alcohol dehydrogenase mutant, genetically engineered bacterium containing recombinant vector, and application of alcohol dehydrogenase mutant and genetically engineered bacterium
ActiveCN112522224AHigh stereoselectivityHigh activityOxidoreductasesGenetic engineeringKetoneEngineered genetic
The invention discloses an alcohol dehydrogenase mutant with improved activity and stereoselectivity, a recombinant vector of alcohol dehydrogenase mutant, a genetically engineered bacterium containing the recombinant vector, and an application of the alcohol dehydrogenase mutant and the genetically engineered bacterium. The alcohol dehydrogenase mutant is mutated by taking wild type alcohol dehydrogenase as shown in SEQ ID NO.1 as a template. The 188th site of the wild type alcohol dehydrogenase plays a key role in improving stereoselectivity, and the 93rd site plays a key role in activity. According to the mutant N188L / Q93L of the alcohol dehydrogenase provided by the invention, (S)-1-(2, 6-dichloro-3-fluorophenyl) ethanol can be prepared by catalyzing 2, 6-dichloro-3-fluoroacetophenonewith high activity and high stereoselectivity, the activity and the stereoselectivity of various ketones are improved, and the mutant N188L / Q93L is more suitable for industrial production and has a wide application prospect.
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation method and application of effective ingredients of rose fragrance emitting agent for cigarettes
InactiveCN101519417AThe reaction steps are simpleShorten the timeSugar derivativesTobacco treatmentSilver carbonateAdditive ingredient
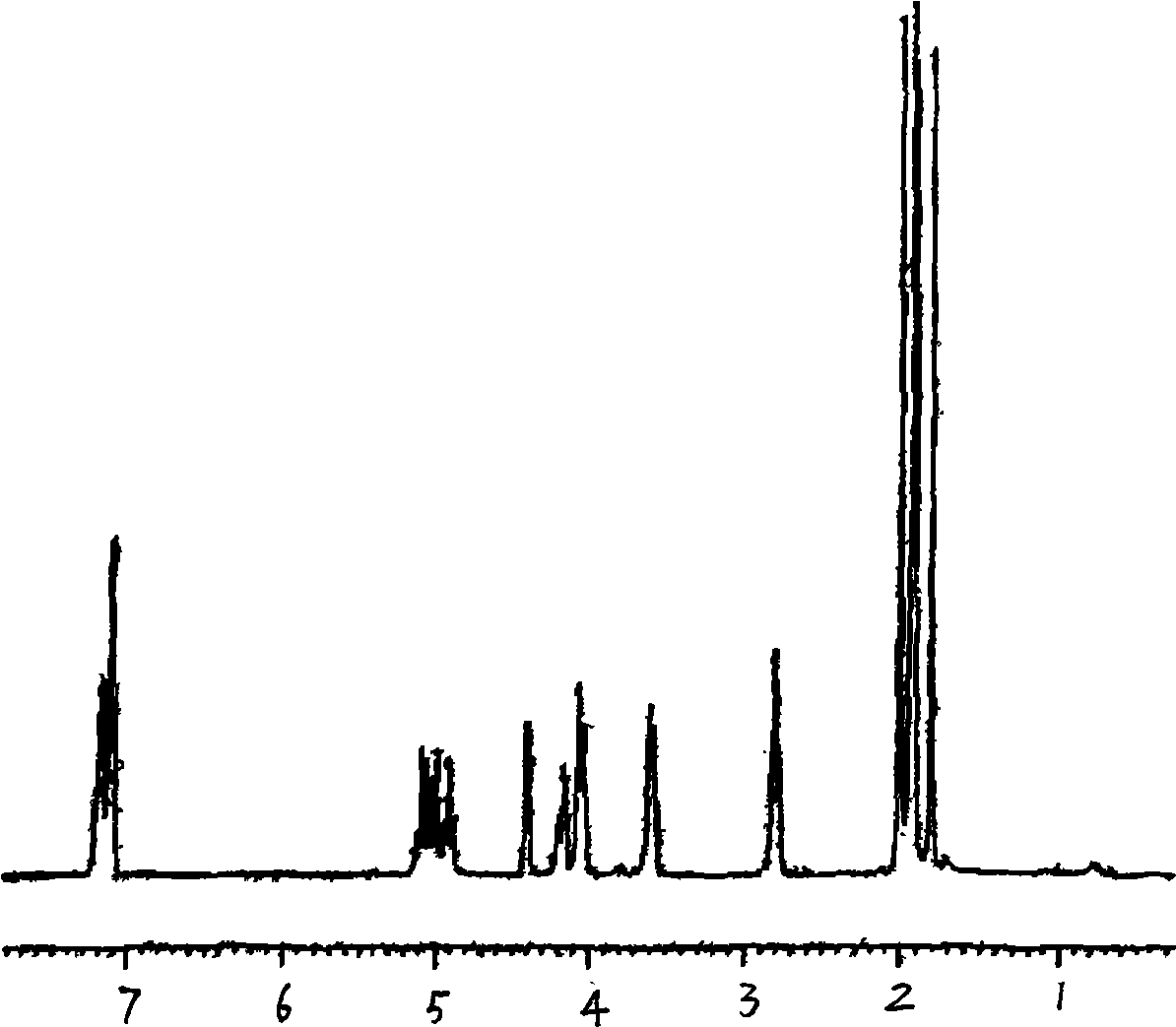
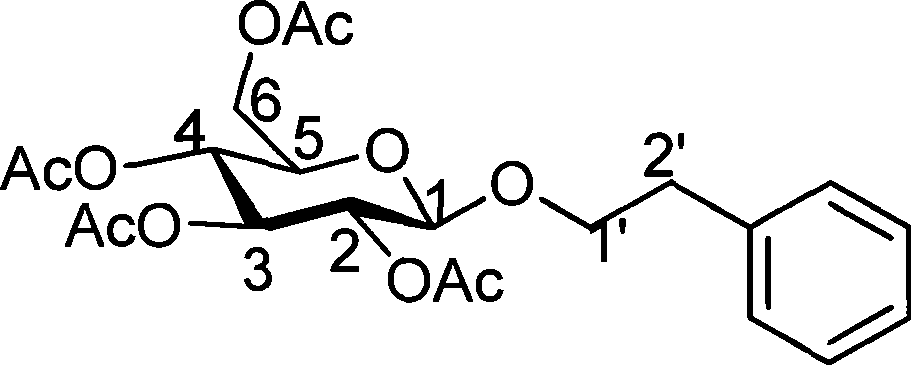
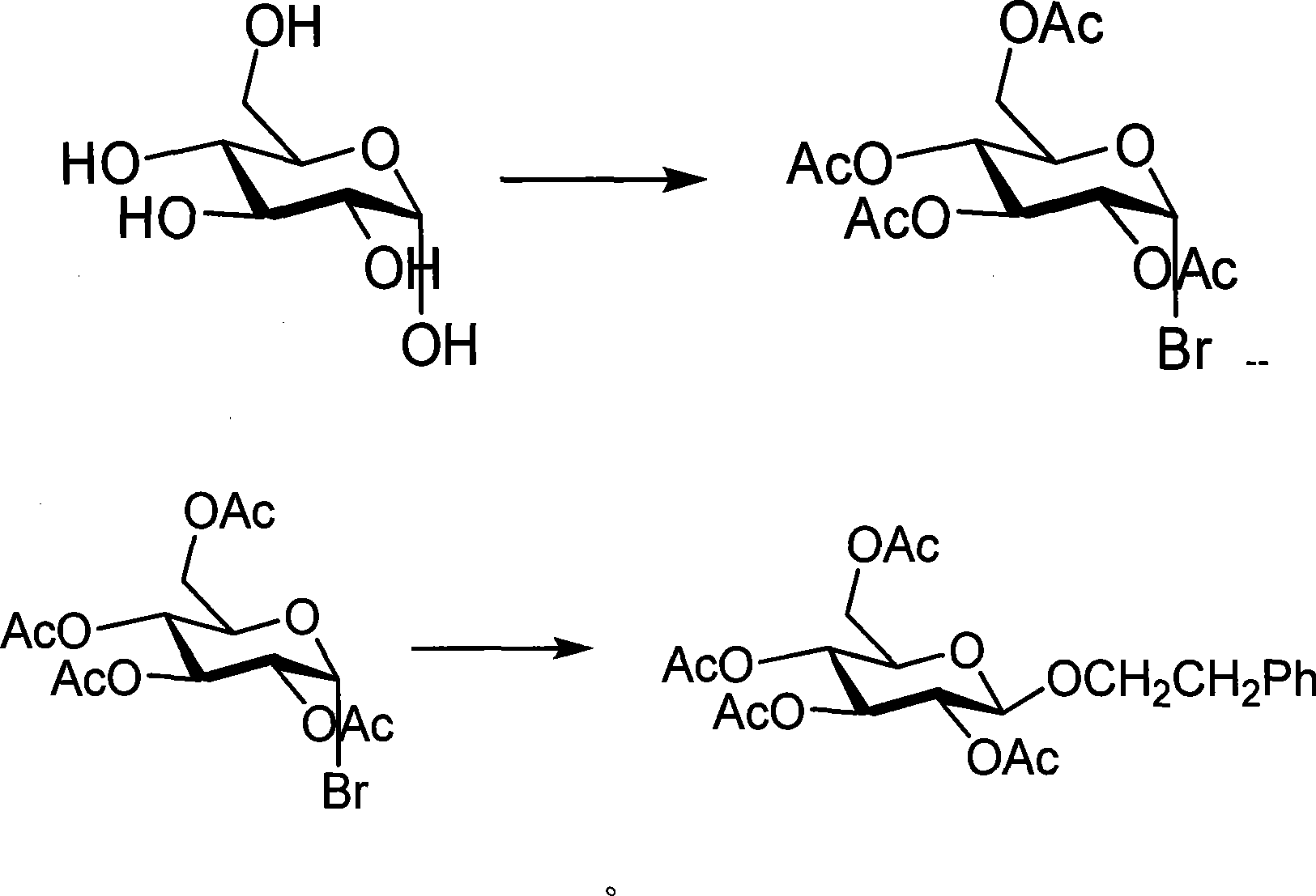
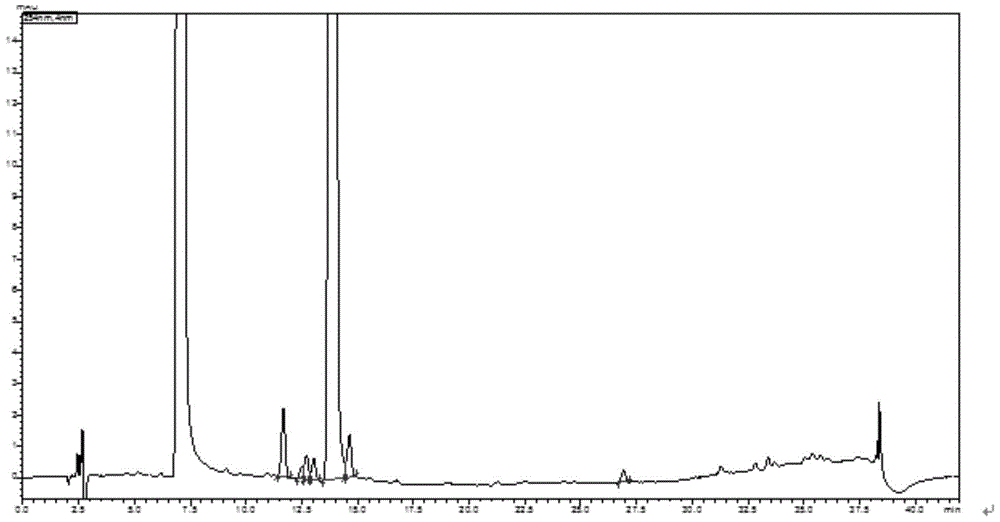
The invention discloses a preparation method and an application of effective ingredients of a rose fragrance emitting agent for cigarettes. The method comprises the following steps: utilizing a Cohen-Kohler reaction to prepare phenylethyl2,3,4,6-4-O-acetyl-beta-D-glucopyranoside; acetylizing glucose under the catalysis of perchloric acid and reacting with hydrogen bromide; removing solvent and recrystallizing in aether to obtain a white crystal of alpha-acetyl-1-bromoglucose; carrying out a photophobic reaction of the white crystal of alpha-acetyl-1-bromoglucose, phenylethyl alcohol and silver carbonate in a ratio of 1:2:1, removing the solvent and purifying by gel chromatography columns to obtain a purified phenylethyl2,3,4,6-4-O-acetyl-beta-D-glucopyranoside product. The prepared phenylethyl2,3,4,6-4-O-acetyl-beta-D-glucopyranoside cracks about 350 DEG C and can emit rose fragrance in main smoke and branch smoke. The prepared rose fragrance emitting agent uses the phenylethyl2,3,4,6-4-O-acetyl-beta-D-glucopyranoside as a main ingredient, can effectively emit objective fragrance ingredients and has the advantages of simple and easily-obtained raw materials, low cost and simple reaction equipment.
Owner:UNIV OF SCI & TECH OF CHINA
Process for preparing knitwear cashmere fluff treating agent
The invention discloses a process for preparing a knitwear cashmere fluff treating agent. The process comprises the following steps: (1) mixing and uniformly stirring the following components in parts by mass: 1-2 parts of hydroxypropyl methyl cellulose, 25-30 parts of ethyl acrylate, 7-8 parts of sodium hexametaphosphate, 25-30 parts of polyoxyethylene octyl phenyl alcohol, 15-17 parts of stearic acid, 2-3 parts of hydroxymethyl acrylamide, 4-5 parts of potassium persulfate and 185-190 parts of water; and (2) adding 5-7 parts by mass of paraffin, 10-15 parts by mass of polypropylene glycol and 6-8 parts by mass of 1,4-butanediol for mixing, heating to the temperature of 50-60 DEG C, stirring so as to completely and uniformly mix the materials, and cooling to room temperature, thus obtaining the finished product. The prepared knitwear cashmere fluff treating agent has a good anti-pilling effect.
Owner:苏州市拉波尼服饰有限公司
Optical stable desonide preparation
InactiveCN106474047ADetection without interferenceWeakening rangeOrganic active ingredientsAerosol deliveryPharmaceutical formulationPhenylethyl Alcohol
The invention belongs to the field of the pharmaceutical preparation, relates to a desonide preparation, and in particular to a more optical stable desonide preparation which uses phenylethyl alcohol and / or benzyl alcohol and / or 2-phenoxyl ethanol and / or sorbic acid as antiseptics of the desonide preparation to replace the antiseptics methyl-p-hydroxybenzoate and propyl p-hydroxybenzoate. Each component in the desonide preparation does not disturb a related substance detection system. Related substances meet control limitations of related substance guiding principles of chemical medicaments, and photosensitiveness of the preparation is obviously improved. In a contrast investigation of illumination influence factors, the related substance degraded amplitude and the content decrease amplitude in the desonide preparation are all obviously reduced, and the product stability is improved significantly.
Owner:CHONGQING HUAPONT PHARMA
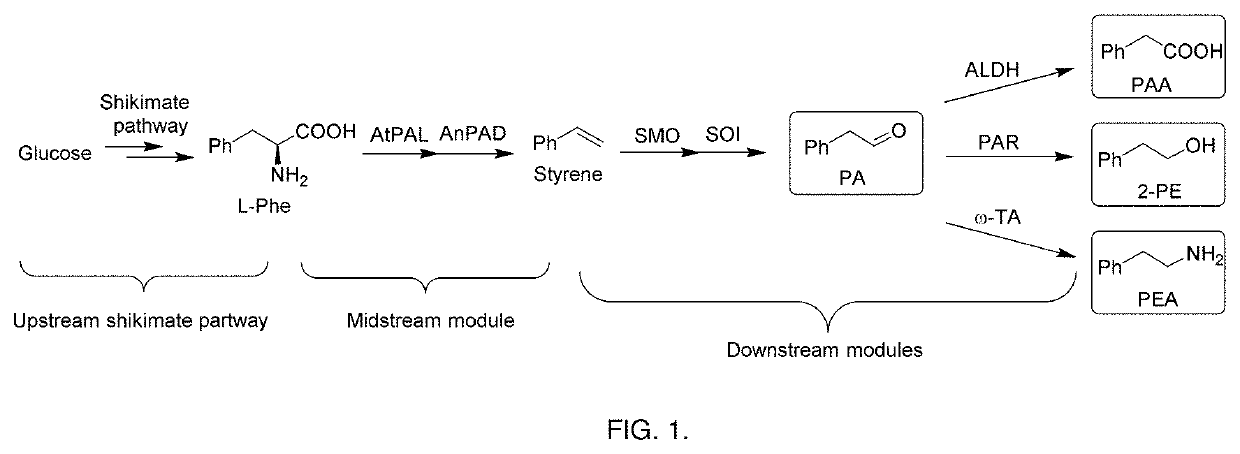
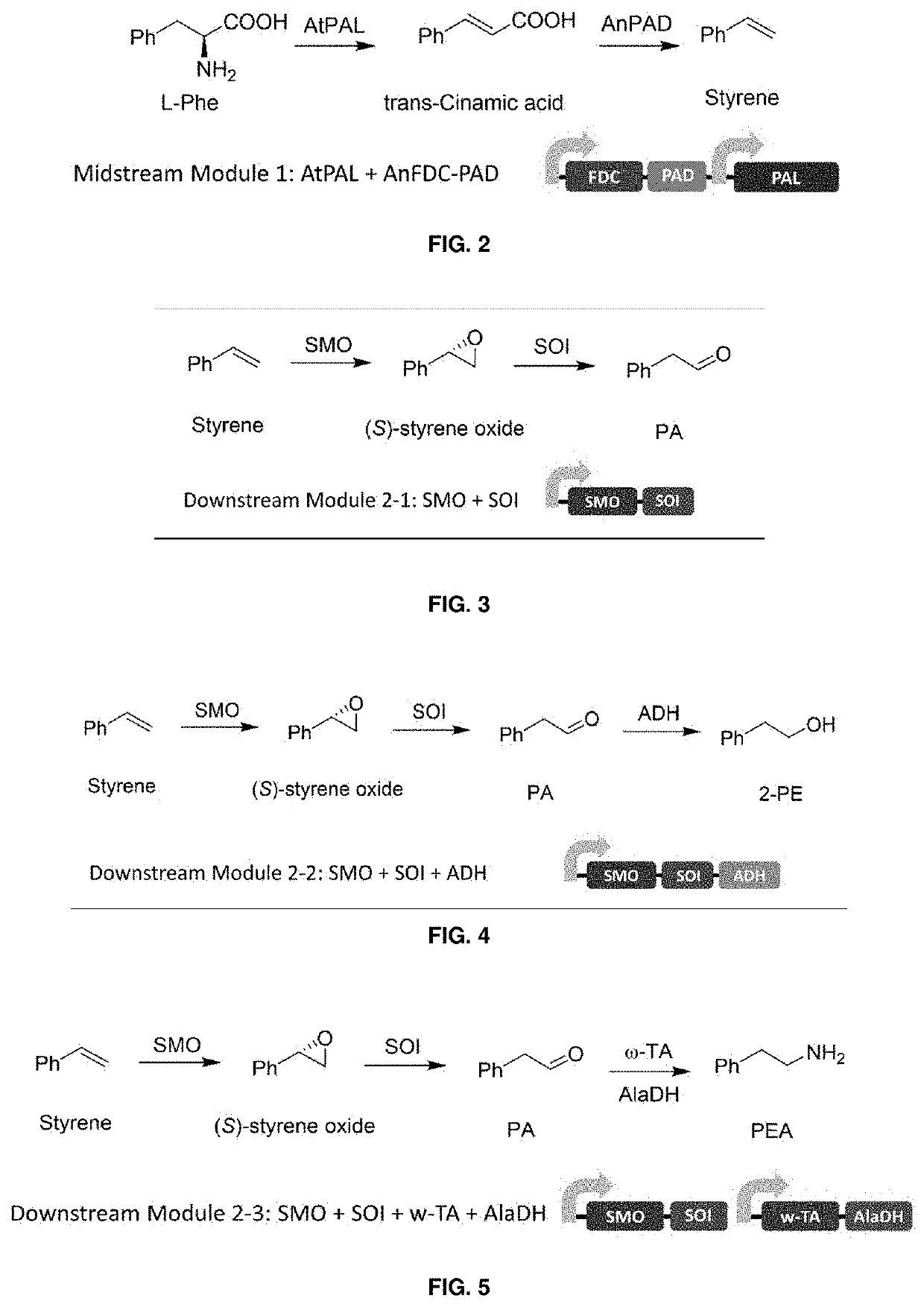
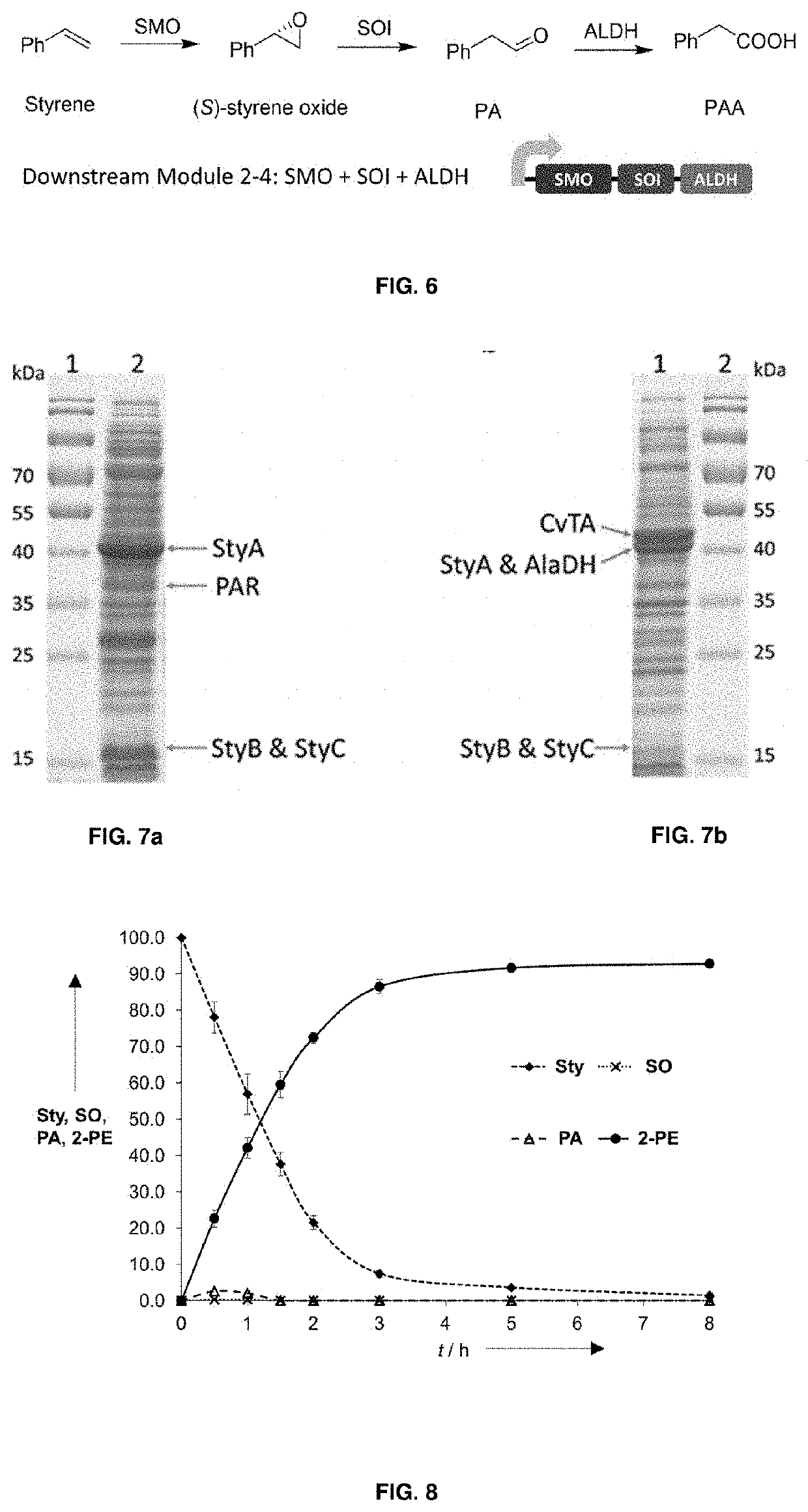
Bioproduction of phenethyl alcohol, aldehyde, acid, amine, and related compounds
This invention relates to the bioproduction of substituted or unsubstituted phenylacetaldehyde, 2-phenylethanol, phenylacetic acid or phenylethylamine by subjecting a starting material comprising glucose, L-phenylalanine, substituted L-phenylalanine, styrene or substituted styrene to a plurality of enzyme catalyzed chemical transformations in a one-pot reaction system, using recombinant microbial cells overexpressing the enzymes. To produce phenylacetaldehyde from styrene, the cells are modified to overexpress styrene monooxygenase (SMO) and styrene oxide isomerase (SOI). To produce phenylacetic acid from styrene, SMO, SOI and aldehyde dehydrogenase are overexpressed. Alternatively, to produce 2-phenylethanol, SMO, SOI and aldehyde reductase or alcohol dehydrogenase are overexpressed, while to produce phenylethylamine, SMO, SOI and transaminase are overexpressed.
Owner:NAT UNIV OF SINGAPORE
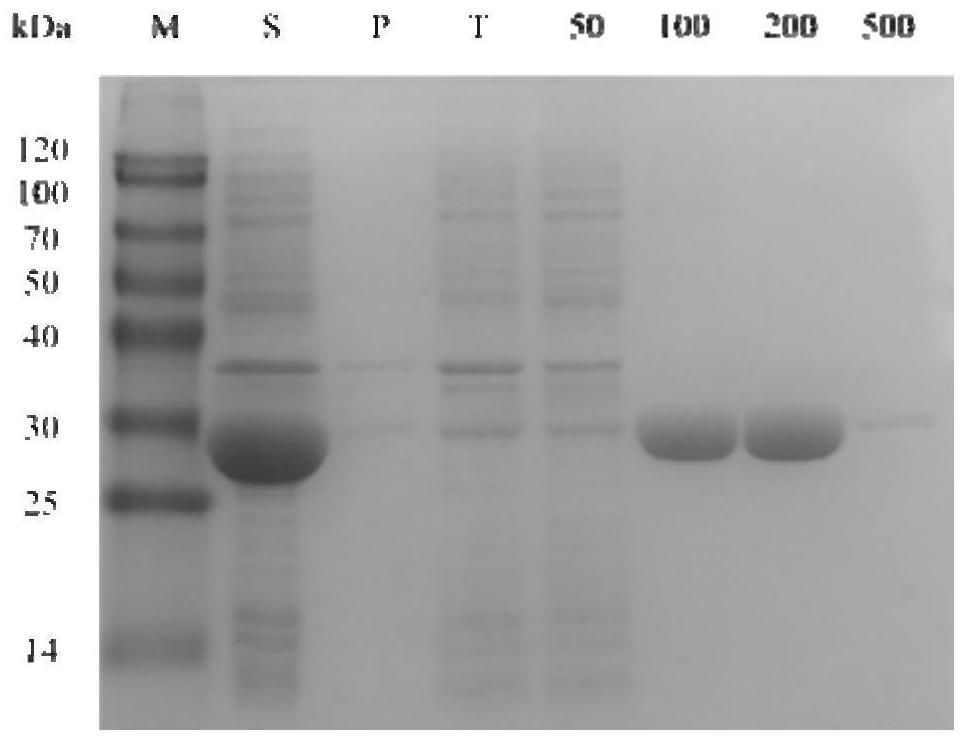
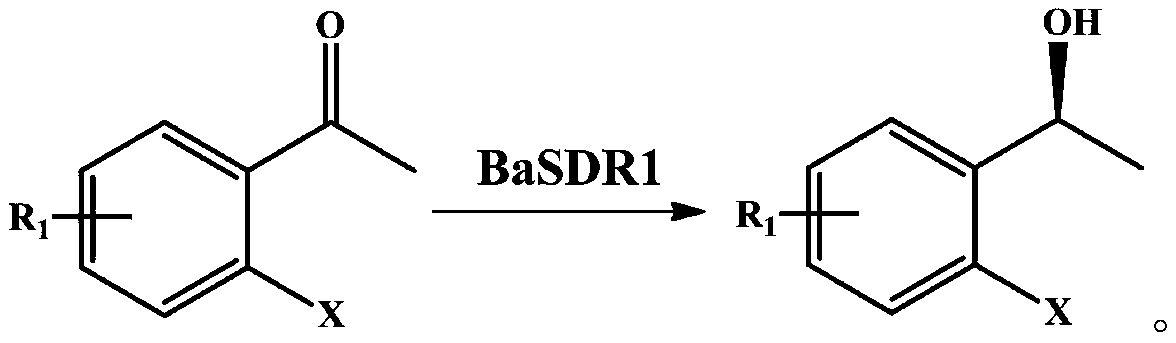
Method for catalyzed synthesis of chiral ortho-halo-alpha-phenyl ethanol by carbonyl reductase
ActiveCN110129382AEasy to synthesizeSimple methodMicrobiological testing/measurementMicroorganism based processesHalogenHydrogen
The invention discloses an application of carbonyl reductase BaSDR1 or engineering bacteria containing carbonyl reductase BaSDR1 in asymmetric reduction of latent chiral ortho-haloacetophenone to prepare chiral ortho-halo-alpha-phenyl ethanol. The latent chiral ortho-haloacetophenone has the general structural formula represented by the formula (I), wherein X is one of F, Cl and Br; R1 is hydrogenor halogen. The chiral alcohol with high optical purity can be synthesized with greenness, high efficiency and high stereoselectivity.
Owner:杭州文德阶生物科技有限公司
Amiotide eye drop containing composite bacteriostatic agent
InactiveCN102204931AGood pollution effectEnhanced inhibitory effectSenses disorderPharmaceutical delivery mechanismEthyl hydroxybenzoatePhenethyl alcohol
The invention discloses an amiotide eye drop containing a composite bacteriostatic agent. The composite bacteriostatic agent is phydroxybenzoate ester and phenethyl alcohol, wherein the concentration of the phydroxybenzoate ester is 0.1 to 0.4g / L; the concentration of the phenethyl alcohol is 4 to 5ml / L; and the phydroxybenzoate ester is one of or a mixture of more than two of methyl parahydroxybenzoate, ethyl p-hydroxybenzoate, propyl p-hydroxybenzoate and butyl p-hydroxybenzoate. Through the combined collaborative bacteriostatic action of the phydroxybenzoate ester and the phenethyl alcohol, the amiotide eye drop can improve the bacteriostatic activity, enhances the microbial contamination resistance, expands the antibacterial spectrum and has relatively high inhabitation effect on gram-negative bacterium, gram-positive bacterium and fungi. The potency assay of the bacteriostatic agent proves that the amiotide eye drop completely accords with the rule of a bacteriostatic agent efficacy test method guiding principle, which is the appendix of 'China Pharmacopeia 2000 (vol. II)', and the safety and the efficiency of the amiotide eye drop can satisfy clinical needs.
Owner:ZHEJIANG UNIV OF TECH +1
Acetophenone and 1-phenethyl alcohol separation method
InactiveCN105418380AHigh mechanical strengthImprove mechanical stabilityHydroxy compound separation/purificationCarbonyl compound separation/purificationPreferential adsorptionPhenethyl alcohol
The invention discloses an acetophenone and 1-phenethyl alcohol separation method. According to the method, the adsorption capacity difference of a soluble starch-immobilized beta-cyclodextrin polymer in adsorbing acetophenone and 1-phenethyl alcohol is used, the soluble starch-immobilized beta-cyclodextrin polymer is taken as an adsorbent, and an aim of separation purification is achieved by preferential adsorption to the acetophenone under a mild condition. The separation method has the advantages of being mild in separation condition, simple in process, simple and convenient in operation, good in separation effect, green and environment-friendly, good in adsorbent regenerability, low in running cost and the like.
Owner:GUANGXI UNIV
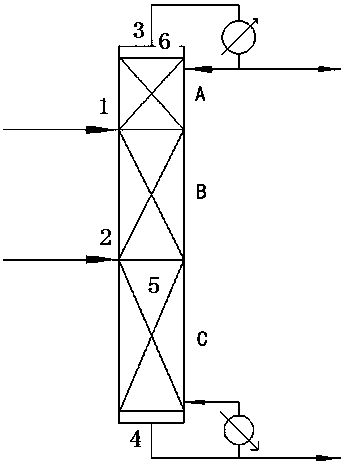
Catalytic rectifying apparatus for splitting chirality 1-phenethyl alcohol by virtue of lipase and method for producing chirality 1-phenethyl
InactiveCN108479100AObvious superiorityReduce contact timeChemical industryEnzyme production/based bioreactorsPhenethyl alcoholTower
The invention relates to a catalytic rectifying apparatus for splitting chirality 1-phenethyl alcohol by virtue of lipase and a method for producing the chirality 1-phenethyl. The catalytic rectifyingapparatus is characterized by comprising a rectifying tower body with a reaction section upper material inlet, a reaction section lower material inlet, a top material outlet and a kettle material outlet, wherein the 1-phenethyl alcohol is introduced from the reaction section upper material inlet, an acyl donor is introduced from the reaction section lower material inlet, a reaction section in therectifying tower is filled with the lipase fixed on the resin and used as a catalyst, and the top material outlet discharges a reaction product fatty alcohol; and a reaction product R-type ester, non-reacted S-type 1-phenethyl alcohol and acyl donor are discharged from the bottom of the tower. By utilizing the apparatus and method of the invention, the reaction conversion rate of 99 percent of R-1-phenethyl alcohol or more can be realized, the R-type ester product with enantiomeric excess greater than 99 percent and the S-1-phenethyl alcohol with the enantiomeric excess greater than 98 percent can be obtained, the production process is greatly simplified, the device size, and the energy consumption and the operation expense are reduced.
Owner:FUZHOU UNIV
Strain and method for producing beta-phenethyl alcohol
ActiveCN108624626APromote growth rateFast growthBacteriaMicroorganism based processesBeta-PhenylethanolEnterobacter sp
The invention provides a strain for producing beta-phenethyl alcohol. The classification name of the strain is Enterobacter sp., and the preservation number of the strain is CGMCC No. 15641. The invention further provides a method using the strain to produce beta-phenethyl alcohol. The method includes: activating the Enterobacter, performing liquid fermentation, and performing tank fermentation; performing concentration extraction on the beta-phenethyl alcohol. Detection shows that beta-phenethyl alcohol yield can reach up to 0.5g / L. The Enterobacter MF024 is good in genetic stability, low inculture cost and the like. The fermentation and extraction method is mild in reaction condition, low in cost, environmentally friendly and the like. The obtained beta-phenethyl alcohol is green and natural and has great industrial application advantages.
Owner:SHANGHAI INST OF TECH
Whole-cell catalyst containing phenylpyruvic acid decarboxylase mutant and application to production of phenethyl alcohol
ActiveCN110438055AIncrease enzyme activityReduced catalytic efficiencyBacteriaMicroorganism based processesBacillus licheniformisPhenethyl alcohol
The invention belongs to the technical field of bioengineering, and particularly relates to a whole-cell catalyst containing a phenylpyruvic acid decarboxylase mutant and application to production ofphenethyl alcohol. According to the hole-cell catalyst containing the phenylpyruvic acid decarboxylase mutant, phenylethanol resistant bacillus licheniformis serve as an expression host, heterologousexpression of the phenylpyruvic acid decarboxylase mutant from lactococcus lactis and alcohol dehydrogenase from the bacillus licheniformis is achieved through free plasmid pHY300PLK, and the recombinant bacillus licheniformis with co-expression of the phenylpyruvic acid decarboxylase and the alcohol dehydrogenase are obtained. L-phenylalanine serves as a substrate, and the phenethyl alcohol is produced through whole-cell catalysis of recombinant bacteria. The advantages that the production cost is low, the production condition is mild, impurities in a transformation system is less, the technology steps are simple, and production operation is safe are achieved.
Owner:HUBEI UNIV
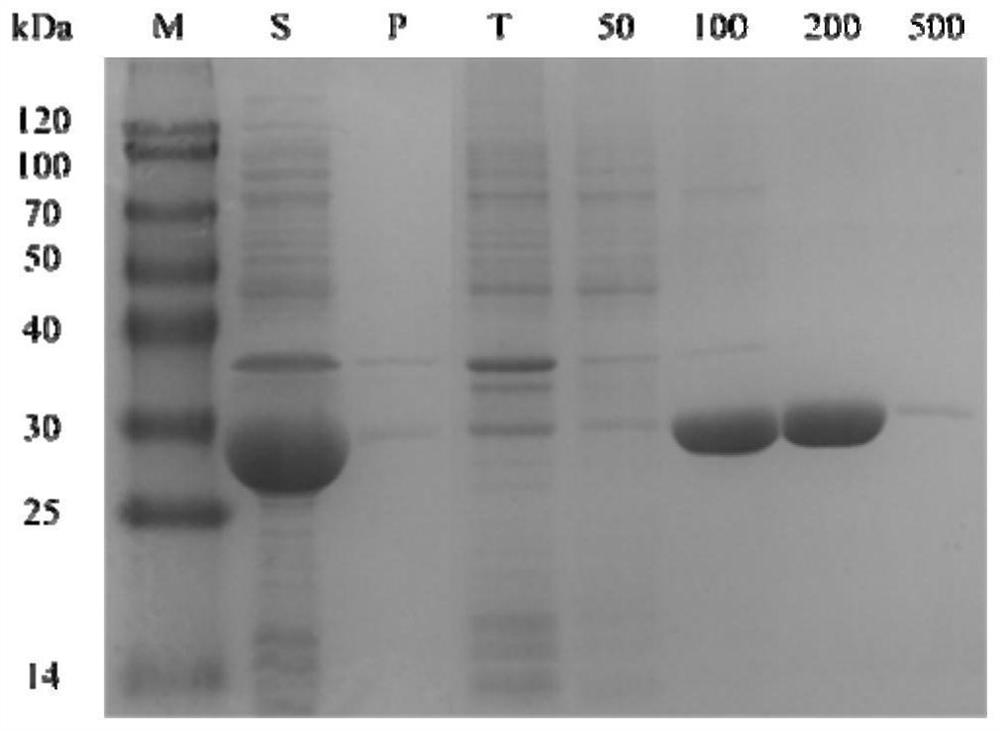
Method for preparing (S)-[3, 5-di (trifluoromethyl) phenyl] ethanol by using Cyberlindnera saturnus
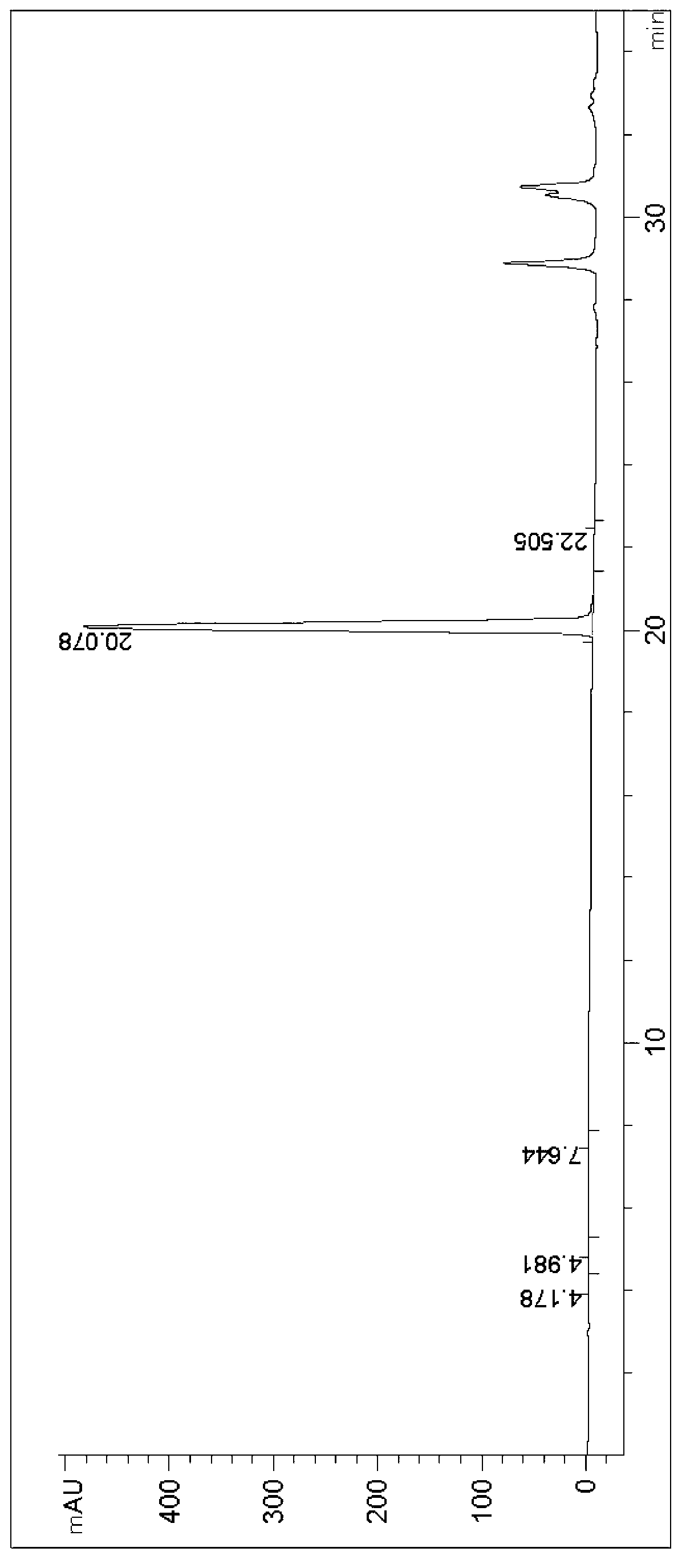
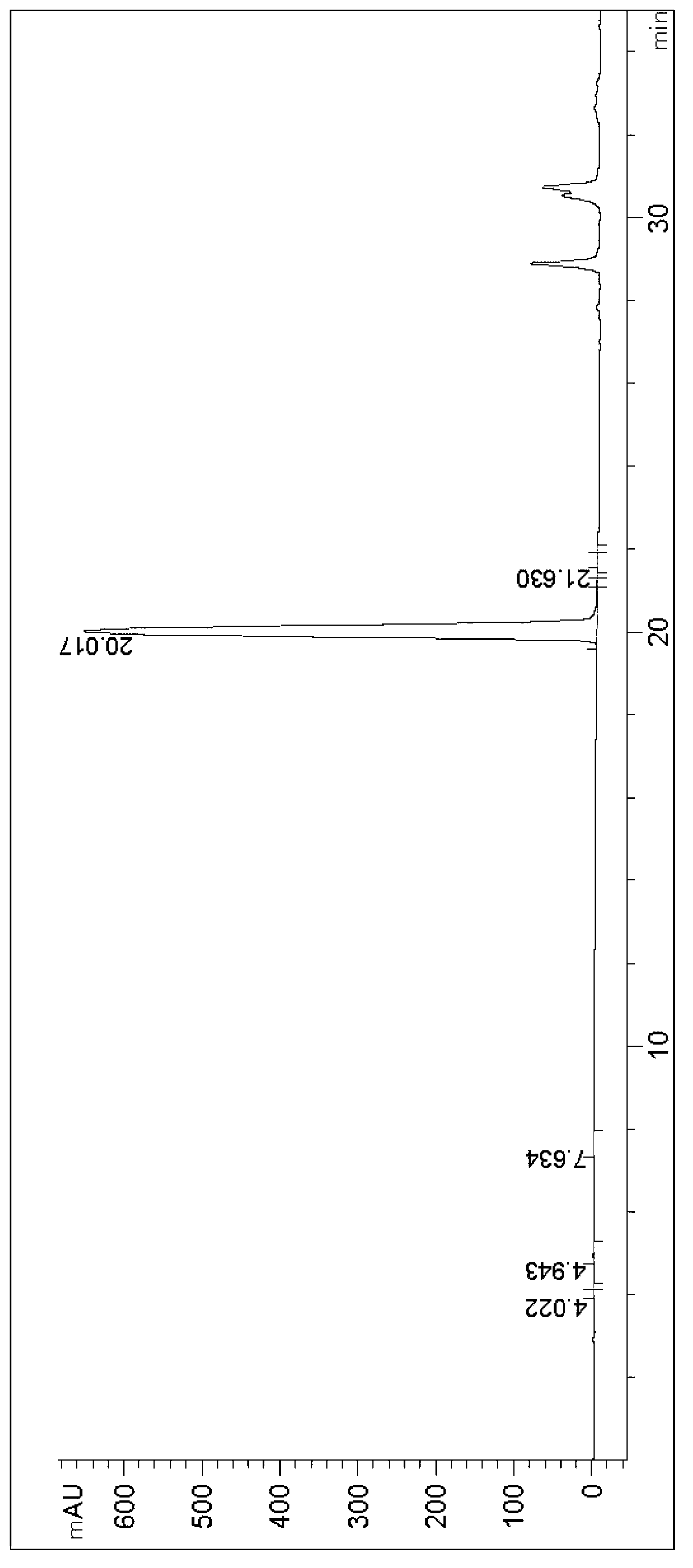
ActiveCN112048538AHigh optical purityFungiMicroorganism based processesPhosphoric acidCyberlindnera saturnus
The invention discloses a method for preparing (S)-[3, 5- di (trifluoromethyl) phenyl] ethanol by using Cyberlindnera saturnus, which comprises the following steps: forming a conversion system in a buffer solution with the pH value of 6.0-8.0 by using wet microbe body obtained by fermentation culture of Cyberlindnera saturnus ZJPH1807 as an enzyme source and 3, 5 bis (trifluoromethyl) acetophenoneas a substrate, and carrying out conversion reaction at 25-45 DEG C at 100-200rpm; after the reaction is finished, separating and purifying a conversion reaction solution to obtain (S)-[3, 5-bis (trifluoromethyl) phenyl] ethanol; in a phosphate buffer solution system with the pH value of 7.5, the addition amount of microbe body is 150 g / L, 128.07 g / L (500 mM) of a substrate is added, conversion is performed for 24 h, and the yield of the product is 80.97%.
Owner:ZHEJIANG UNIV OF TECH
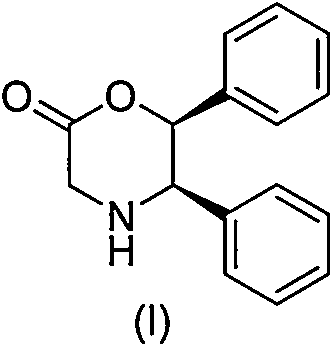
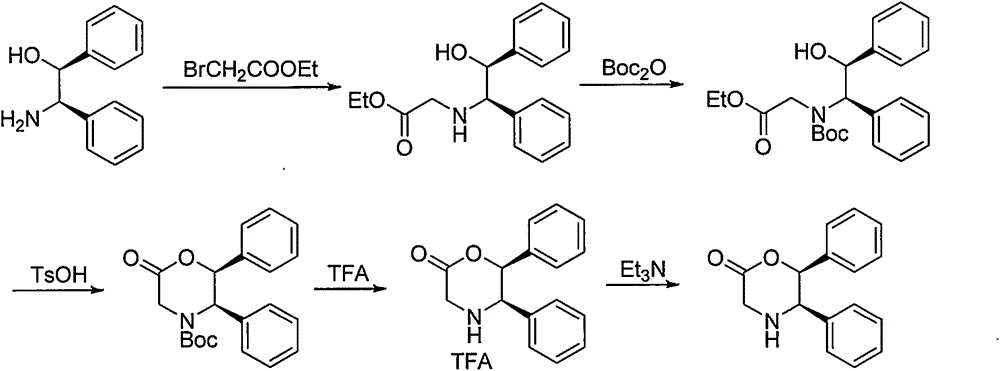
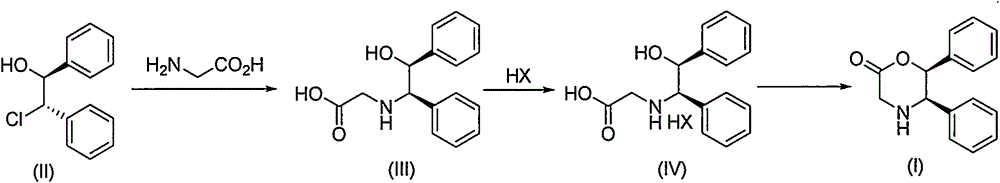
Novel preparation method of (5R, 6S)-5,6-dephenyl -2-morpholinone
InactiveCN104151259ASuitable for industrial productionOrganic compound preparationAmino-carboxyl compound preparationGlycineAcetic acid
The invention discloses a novel preparation method of (5R, 6S)-5,6-dephenyl -2-morpholinone (I). The novel preparation method comprises the following reaction steps: enabling (1S, 2S)-2-chloro-1, 2-diphenylethanol (II) to react with glycine to generate 2-[(1R, 2S)-2-hydroxyl-1,2-diphenylethylamino] acetic acid (III); salifying the compound (III) and acid to form 2-[(1R, 2S)-2-hydroxyl-1,2-diphenylethylamino] ethanoic acid salt (IV); and enabling the compound (IV) to be subjected to ring closing reaction under the action of a ring closing reagent to obtain (5R,6S)-5,6-dephenyl -2-morpholinone (I).
Owner:SHANGHAI AOBO PHARMTECH INC LTD
Anti-pilling agent for wool fabric and preparation method of anti-pilling agent
ActiveCN104195830AImprove pilling resistanceHydrophilicity unchangedFibre treatmentPolymer scienceStearic acid
The invention provides an anti-pilling agent for a wool fabric. The anti-pilling agent consists of the following materials in parts by mass: 15-20 parts of ethyl acrylate, 15-20 parts of polyoxyethylene octyl phenyl alcohol, 10-15 parts of zinc nitrate, 5-8 parts of stearic acid, 5-8 parts of sodium sulfate, 2-4 parts of n-butyl alcohol, 2-4 parts of potassium tartrate, 1-3 parts of chitosan, 2-4 parts of sodium dihydrogen phosphate, 70-80 parts of ethyl alcohol and 320-360 parts of water. The invention also provides a preparation method of the anti-pilling agent. By virtue of the anti-pilling agent, the anti-pilling grade of the wool fabric can be enhanced by over two levels and the hydrophilicity can be kept the same fundamentally.
Owner:汕头市澄海区瑞胜毛织有限公司
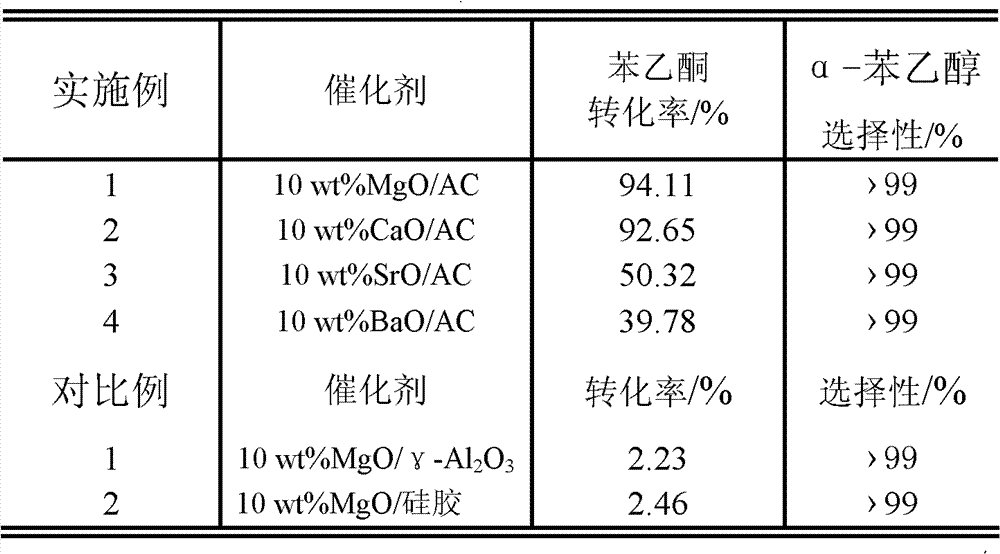
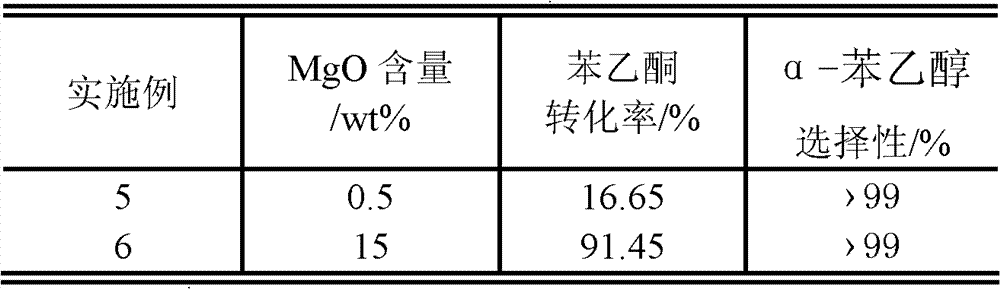
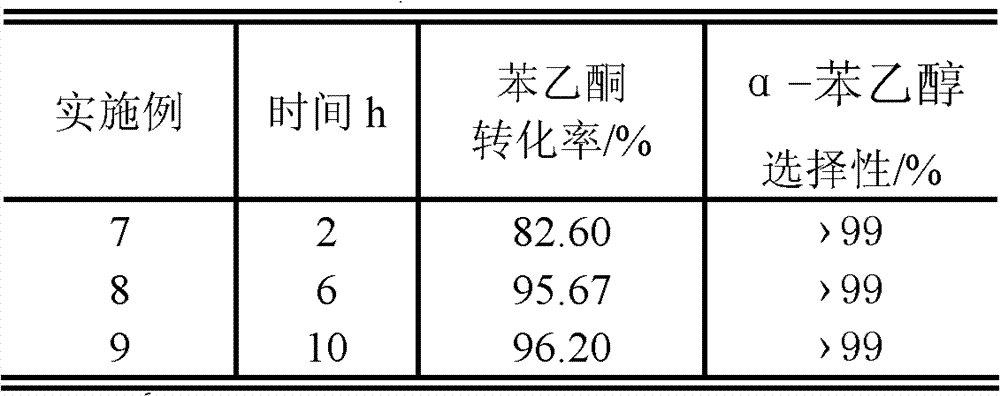
Method for preparing alpha-phenethyl alcohol
ActiveCN101792368BRelaxed reaction conditionsEfficient responseOrganic compound preparationHydroxy compound preparationReaction temperaturePhenethyl alcohol
The invention discloses a method for preparing alpha-phenethyl alcohol. The method comprises the following steps: reacting acetyl benzene serving as the raw material, alcohol serving as a hydrogen source and supported alkaline earth metallic oxide as a catalyst for 1 to 10 hours at a the reaction temperature of between 60 and 130 DEG C; and after the reaction is finished, processing the reaction solution to obtain alpha-phenethyl alcohol, wherein the alcohol is C1 to C7 fatty alcohol or C3 to C7 alicyclic alcohol; the mole ratio of the acetyl benzene to the alcohol is 1:5-30; the supported alkaline earth metallic oxide consists of a carrier and alkaline earth metallic oxide supported on the carrier; the alkaline metallic earth oxide is MgO, CaO, SrO or BaO; the carrier is active carbon; and the supporting capacity of the alkaline earth metallic oxide is between 0.5 and 15 weight percent based on the mass of the carrier. The method for preparing the alpha-phenethyl alcohol has the advantages of mild required reaction condition, high activity and high selectivity of the catalyst, short reaction time, no other by-products in the reaction and no pollution to the environment, and is anenvironmental-friendly green synthetic route.
Owner:日照新睿招商发展有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
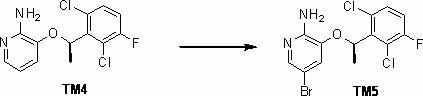
![S-(-)-1-{4-[2-(allyloxy)-ethyl]phenoxy}-3-isopropylamino propan-2-ol, process for preparation thereof and process for preparation of S-(-)betaxolo S-(-)-1-{4-[2-(allyloxy)-ethyl]phenoxy}-3-isopropylamino propan-2-ol, process for preparation thereof and process for preparation of S-(-)betaxolo](https://images-eureka.patsnap.com/patent_img/5cb0837c-51a6-4a83-93d4-99f65b85117c/US06989465-20060124-C00001.png)
![S-(-)-1-{4-[2-(allyloxy)-ethyl]phenoxy}-3-isopropylamino propan-2-ol, process for preparation thereof and process for preparation of S-(-)betaxolo S-(-)-1-{4-[2-(allyloxy)-ethyl]phenoxy}-3-isopropylamino propan-2-ol, process for preparation thereof and process for preparation of S-(-)betaxolo](https://images-eureka.patsnap.com/patent_img/5cb0837c-51a6-4a83-93d4-99f65b85117c/US06989465-20060124-C00002.png)
![S-(-)-1-{4-[2-(allyloxy)-ethyl]phenoxy}-3-isopropylamino propan-2-ol, process for preparation thereof and process for preparation of S-(-)betaxolo S-(-)-1-{4-[2-(allyloxy)-ethyl]phenoxy}-3-isopropylamino propan-2-ol, process for preparation thereof and process for preparation of S-(-)betaxolo](https://images-eureka.patsnap.com/patent_img/5cb0837c-51a6-4a83-93d4-99f65b85117c/US06989465-20060124-C00003.png)
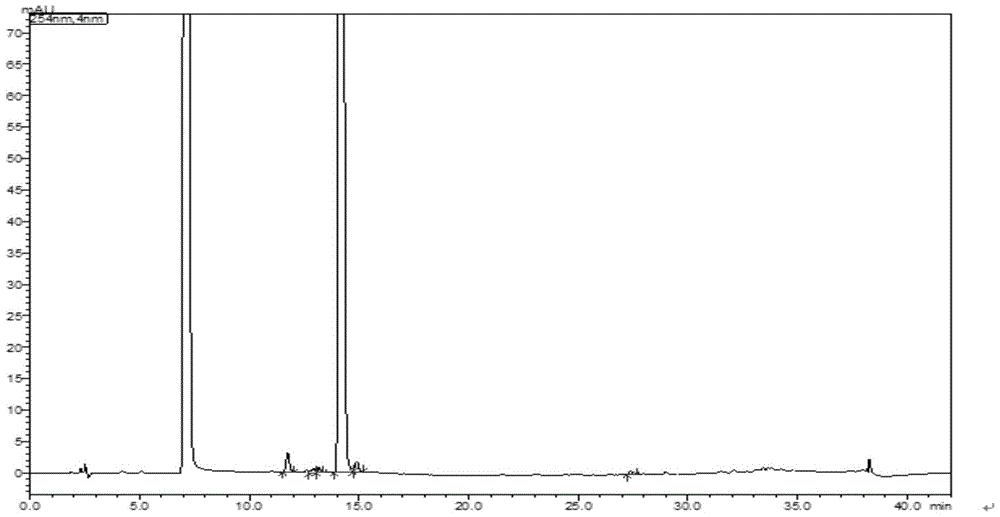




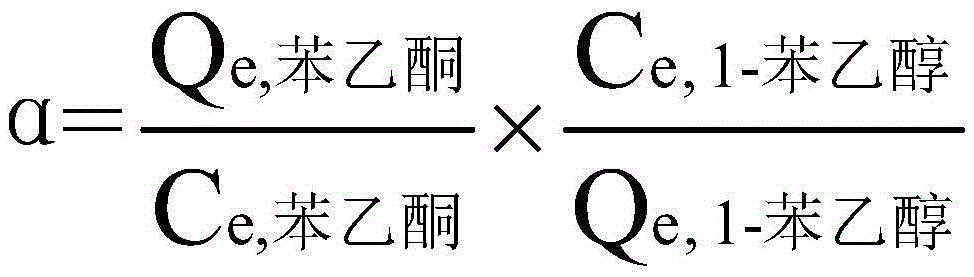

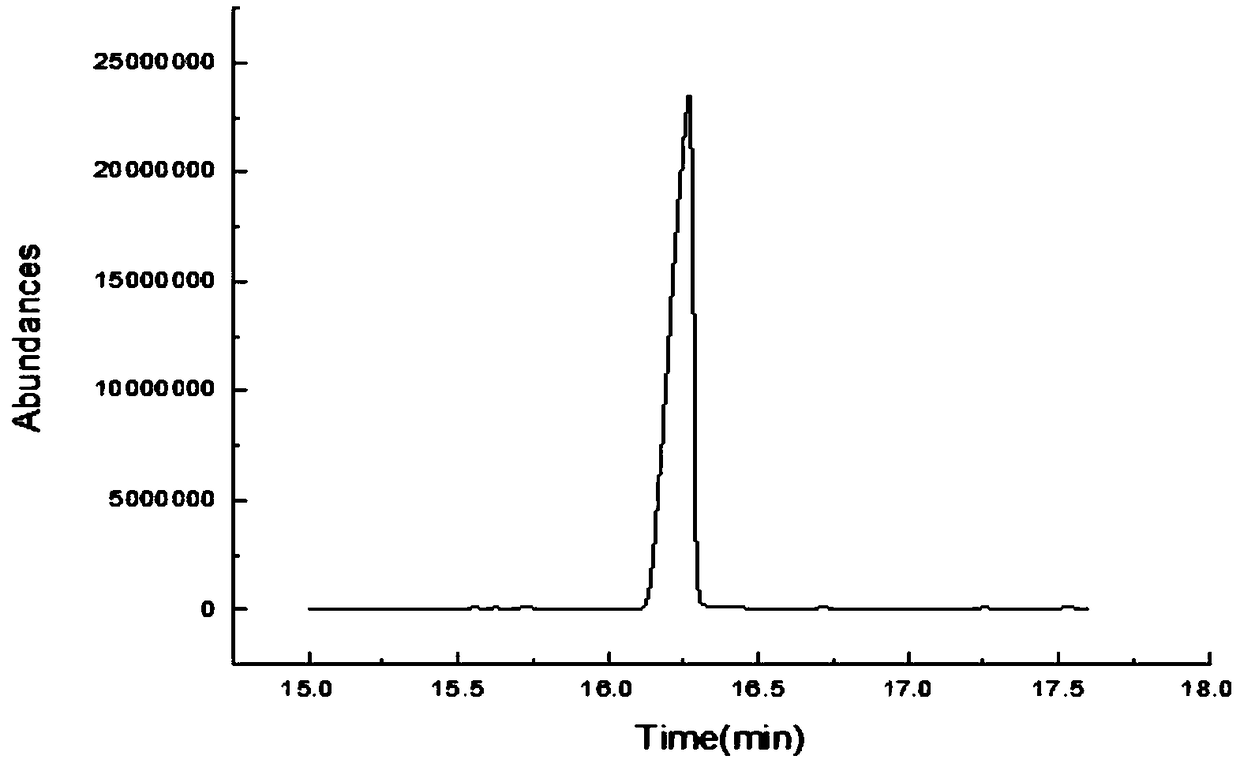
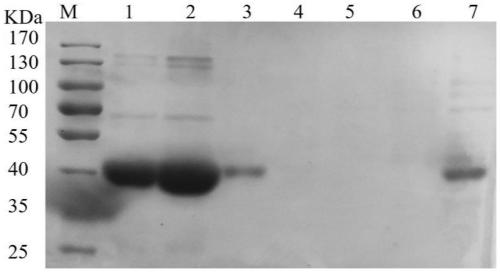
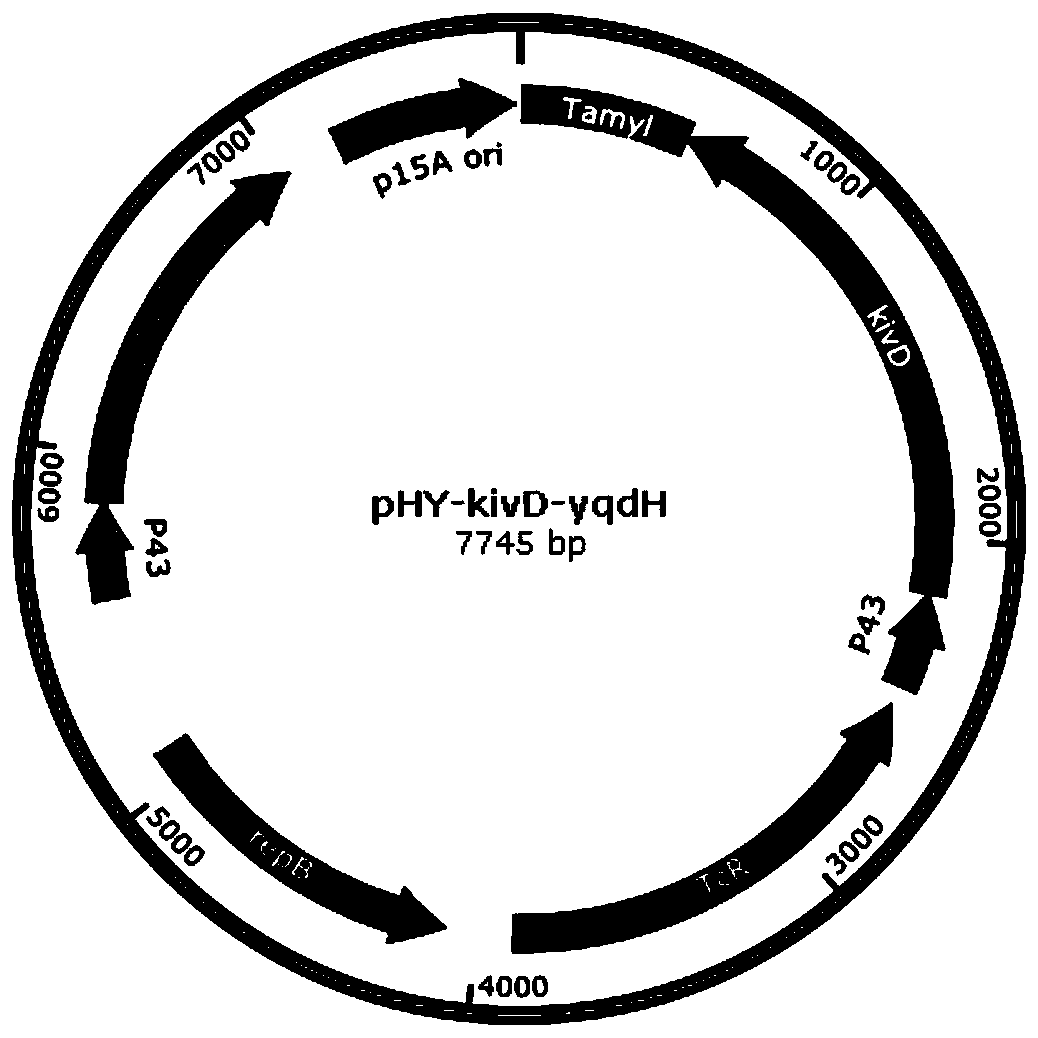
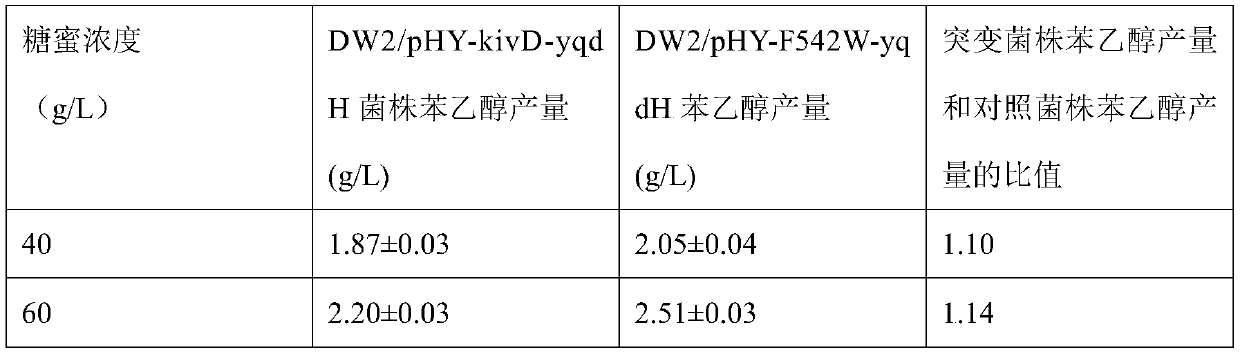
![Method for preparing (S)-[3, 5-di (trifluoromethyl) phenyl] ethanol by using Cyberlindnera saturnus Method for preparing (S)-[3, 5-di (trifluoromethyl) phenyl] ethanol by using Cyberlindnera saturnus](https://images-eureka.patsnap.com/patent_img/e3cb5f6d-37d5-448c-bcea-7a0155a507d6/HDA0002644025370000011.png)
![Method for preparing (S)-[3, 5-di (trifluoromethyl) phenyl] ethanol by using Cyberlindnera saturnus Method for preparing (S)-[3, 5-di (trifluoromethyl) phenyl] ethanol by using Cyberlindnera saturnus](https://images-eureka.patsnap.com/patent_img/e3cb5f6d-37d5-448c-bcea-7a0155a507d6/HDA0002644025370000012.png)
![Method for preparing (S)-[3, 5-di (trifluoromethyl) phenyl] ethanol by using Cyberlindnera saturnus Method for preparing (S)-[3, 5-di (trifluoromethyl) phenyl] ethanol by using Cyberlindnera saturnus](https://images-eureka.patsnap.com/patent_img/e3cb5f6d-37d5-448c-bcea-7a0155a507d6/BDA0002644025330000041.png)