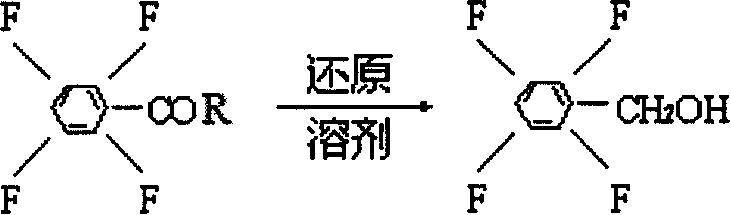
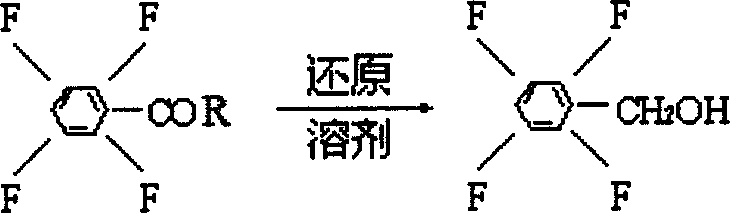
Process preparing 2,3,5,6-phyenyl methanol tetrafluoride
A technology of tetrafluorobenzyl alcohol and tetrafluorobenzoyl chloride, applied in 2, can solve problems such as production and use restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] In a 500ml four-neck flask, add 0.1mol tetrafluorobenzoyl chloride, directly add 300ml tetrahydrofuran, add 38gNaBH at low temperature 4 , 2g cocatalyst, slowly heat up, control the temperature at 58°C, and react for 8 hours. Cool down and filter with suction. Slowly pour the filtrate into 6% hydrochloric acid solution with stirring to keep the solution acidic. After fully stirring, the organic layer was separated and washed with water to be neutral. The solvent was removed under reduced pressure to obtain 172.7 g of tetrafluorobenzyl alcohol with a purity of 99% and a yield of 95%.
Embodiment 2
[0016] In a 500ml four-neck flask, add 0.1mol tetrafluorobenzoyl chloride, directly add 300ml tetrahydrofuran, add 38gNaBH at low temperature 4 , 10g cocatalyst, and pass into nitrogen protection. Slowly raise the temperature, control the temperature at 86°C, and react for 6 hours. Cool down and filter with suction. Slowly pour the filtrate into 10% hydrochloric acid solution with stirring to keep the solution acidic. After fully stirring, the organic layer was separated and washed with water to be neutral. The solvent was removed under reduced pressure to obtain 176 g of tetrafluorobenzyl alcohol with a purity of 99% and a yield of 96.8%.
Embodiment 3
[0018] In a 500ml four-neck flask, add 0.1mol tetrafluorobenzoyl chloride, directly add 300ml tetrahydrofuran, add 38gNaBH at low temperature 4 , 8g cocatalyst, and pass into nitrogen protection. Slowly raise the temperature, control the temperature at 65°C, and react for 7 hours. Cool down and filter with suction. Slowly pour the filtrate into 8% hydrochloric acid solution with stirring to keep the solution acidic. After fully stirring, the organic layer was separated and washed with water to be neutral. The solvent was removed under reduced pressure to obtain 174.9 g of tetrafluorobenzyl alcohol with a purity of 99% and a yield of 96.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com