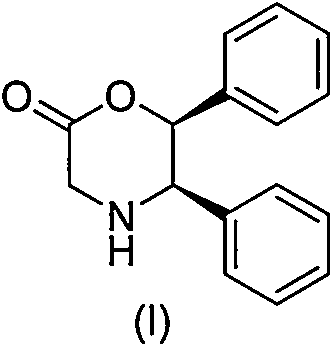
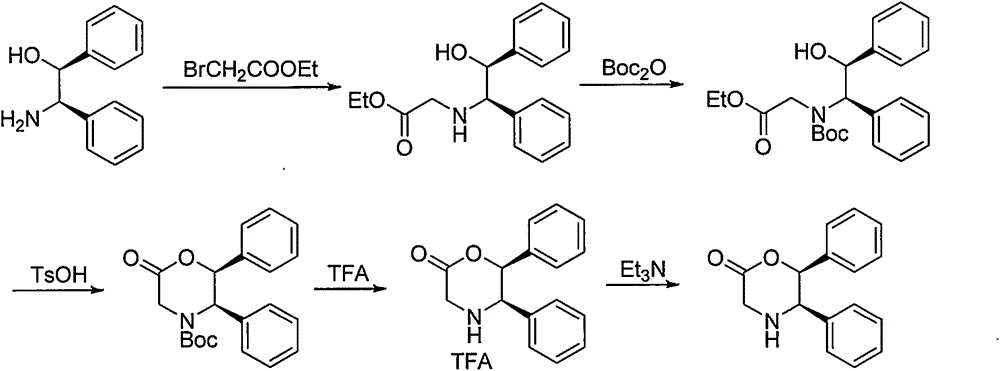
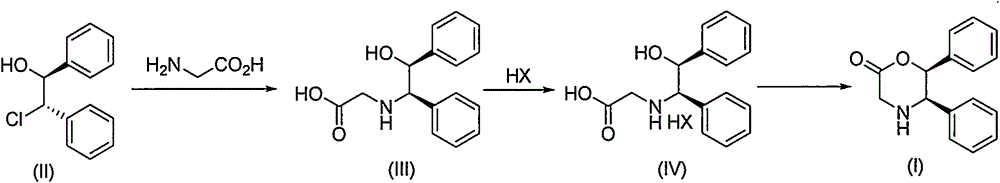
Novel preparation method of (5R, 6S)-5,6-dephenyl -2-morpholinone
A technology of diphenylmorpholine and a new method, applied in the field of preparation-5, can solve the problems of harmfulness to human body and the environment, many steps, increased cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Synthesis of 2-[(1R, 2S)-2-hydroxyl-1,2-diphenylethylamino]acetic acid (III)
[0034] At room temperature, 1860 g of (1S,2S)-2-chloro-1,2-diphenylethanol (II), 258 g of tetrabutylammonium bromide, 890 g of triethylamine and 5 L of tetrahydrofuran were mixed together. Cool down to 0°C, and slowly add 660g of glycine dissolved in 5L of water dropwise under sufficient stirring. After the dropwise addition, tetrahydrofuran was removed under reduced pressure, and 1N hydrochloric acid was added dropwise to the remaining aqueous phase until the Ph of the system was neutral, and solids were precipitated. Filter and dry to obtain 1600g of 2-[(1R,2S)-2-hydroxy-1,2-diphenylethylamino]acetic acid (III), which is directly carried out to the next reaction.
Embodiment 2
[0035] Example Two Synthesis of 2-[(1R, 2S)-2-hydroxyl-1,2-diphenylethylamino]acetic acid hydrochloride (IVa)
[0036] At room temperature, 200 g of 2-[(1R,2S)-2-hydroxy-1,2-diphenylethylamino]acetic acid (III) obtained in Example 1 and 400 mL of ethanol were mixed with each other. Then slowly add 360 mL of 35% hydrogen chloride ethanol solution. The mixture was fully stirred at 40°C for 12h, cooled and filtered. After the solid was fully washed with ethanol, it was filtered and dried to obtain 210 g of 2-[(1R,2S)-2-hydroxy-1,2-diphenylethylamino]acetic acid hydrochloride (IVa) as a white solid.
Embodiment 3
[0037] Embodiment three (5R, 6S)-5, the synthesis of 6-diphenylmorpholin-2-ketone (I)
[0038]Stir 400g of thionyl chloride with 10mL of N,N-dimethylformamide and 2L of dichloromethane. Then add 210 g of 2-[(1R,2S)-2-hydroxy-1,2-diphenylethylamino]acetic acid hydrochloride (IVa) obtained in Example 2, raise the temperature to 40°C, and react for 24 hours . Thionyl chloride and dichloromethane were distilled off under reduced pressure, and the residue was added to 2L of ethyl acetate, washed with saturated sodium bicarbonate solution, and separated to obtain an organic phase. After the organic phase was washed with water and dried over anhydrous sodium sulfate, the ethyl acetate was removed under reduced pressure, and when 60% of the ethyl acetate was removed, the reduced pressure was stopped and left to stand at room temperature. After sufficient crystallization, 135 g of (5R,6S)-5,6-diphenylmorpholin-2-one (I) was obtained by filtration as a white solid with a purity of 99....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com