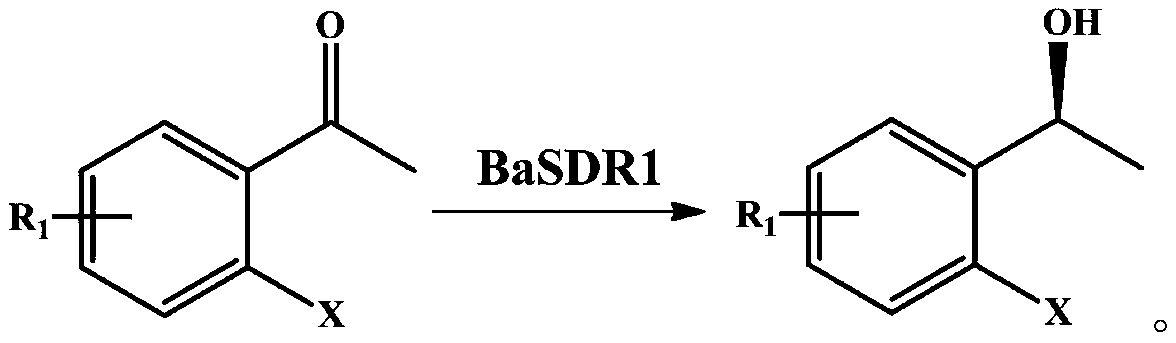
Method for catalyzed synthesis of chiral ortho-halo-alpha-phenyl ethanol by carbonyl reductase
A technology of carbonyl reductase and reductase, which is applied in the direction of microorganism-based methods, oxidoreductase, biochemical equipment and methods, etc., can solve the problems that cannot meet the needs of the synthesis of chiral ortho-halo-α-phenylethanol, which is rare and other issues, to achieve the effects of good industrial application development prospects, environmental friendliness, and wide substrate adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
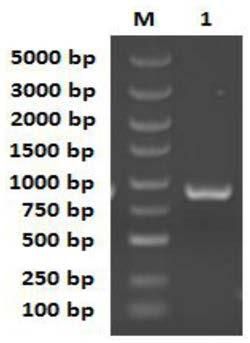
[0041] The preparation method of the carbonyl reductase BaSDR1 gene is as follows: the nucleotides shown in SEQ ID No: 3 and the nucleotides shown in SEQ ID No: 4 are respectively used as upstream and downstream primers, and the genomic DNA of Bacillus aryabhattai NWPU-1801 is used as a template. Gene amplification was carried out by PCR, and the full-length 885bp carbonyl reductase gene sequence was obtained.
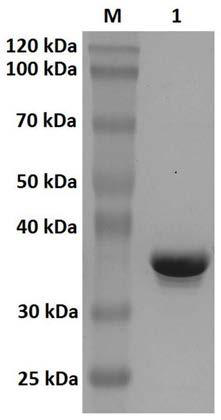
[0042] The coding gene of the above-mentioned carbonyl reductase BaSDR1 is carried by a recombinant expression vector. These recombinant vectors can be constructed by linking the nucleotide sequence encoding carbonyl reductase BaSDR1 of the present invention to various vectors by conventional methods in the art. The vector can be various conventional vectors in the art, such as various plasmids, phage or virus vectors, etc., preferably pET-30a. Preferably, the recombinant expression vector of the present invention can be obtained by the following method: the carbonyl ...
Embodiment 1
[0046] Embodiment 1: Isolation and identification of bacterial strain Bacillus aryabhattaiNWPU-1801:
[0047] (1) Separation:
[0048] Add 1.0g of local soil samples from Xi'an to 9.0mL of 0.85% normal saline, oscillate fully on a vortex shaker to form a uniform soil suspension, and let it stand for 10min; In a 250mL Erlenmeyer flask with enriched medium, place it in a shaker at 30°C and 220rpm for 24h, then transfer 1.0mL of the culture solution to a fresh enriched medium, and continue to cultivate for 24h; carry out three rounds of enrichment in this way , Dilute the enriched culture solution into multiple gradients and spread it on the plate screening medium, culture it in a 30°C incubator for 24 hours, and obtain a single colony;
[0049] The enrichment medium uses 1.0g / L 2'-fluoroacetophenone as the sole carbon source, and other components are as follows, expressed in final concentration: (NH 4 ) 2 SO 4 5.0g / L, MgSO 4 ·7H 2 O 0.5g / L, NaCl 1.0g / L, K 2 HPO 4 2.0g / L,...
Embodiment 2
[0054] Example 2: Acquisition of gene encoding carbonyl reductase BaSDR1, construction of recombinant plasmid and transformation of Escherichia coli:
[0055] Use a DNA extraction kit to extract the whole genome DNA of Bacillus aryabhattaiNWPU-1801 cells, use the extracted total cell DNA as a template, and use the upstream primer GCTGA GGATCC ATGTCAAAGTTAAATAATCC and downstream primer GCATC CTCGAG TTAAGCTAGTTTCTATTCCGC was used as primer for PCR amplification reaction. The total volume of the PCR reaction system is 50 μL, and the amount of each component added is: 5×PrimeSTAR TM10 μL of HS DNA polymerase buffer, 4 μL of 10 mM dNTP mixture, including: 2.5 mM each of dATP, dCTP, dGTP and dTTP, 1 μL of each upstream primer and downstream primer at a concentration of 50 μM, 1 μL of genomic DNA, PrimeSTAR TM HS DNApolymerase 0.5μL, nucleic acid-free water 32.5μL. The PCR reaction conditions were as follows: pre-denaturation at 98°C for 1 min, followed by a temperature cycle o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com