Alcohol dehydrogenase mutant with improved activity and stereoselectivity, recombinant vector of alcohol dehydrogenase mutant, genetically engineered bacterium containing recombinant vector, and application of alcohol dehydrogenase mutant and genetically engineered bacterium
A stereoselective, alcohol dehydrogenase technology, applied in the field of protein engineering, can solve the problems of limited application of alcohol dehydrogenase, stereoselectivity and low activity substrate inhibition and product inhibition, etc., to achieve activity and stereoselectivity The effect of improving property, high stereoselectivity, and improving stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
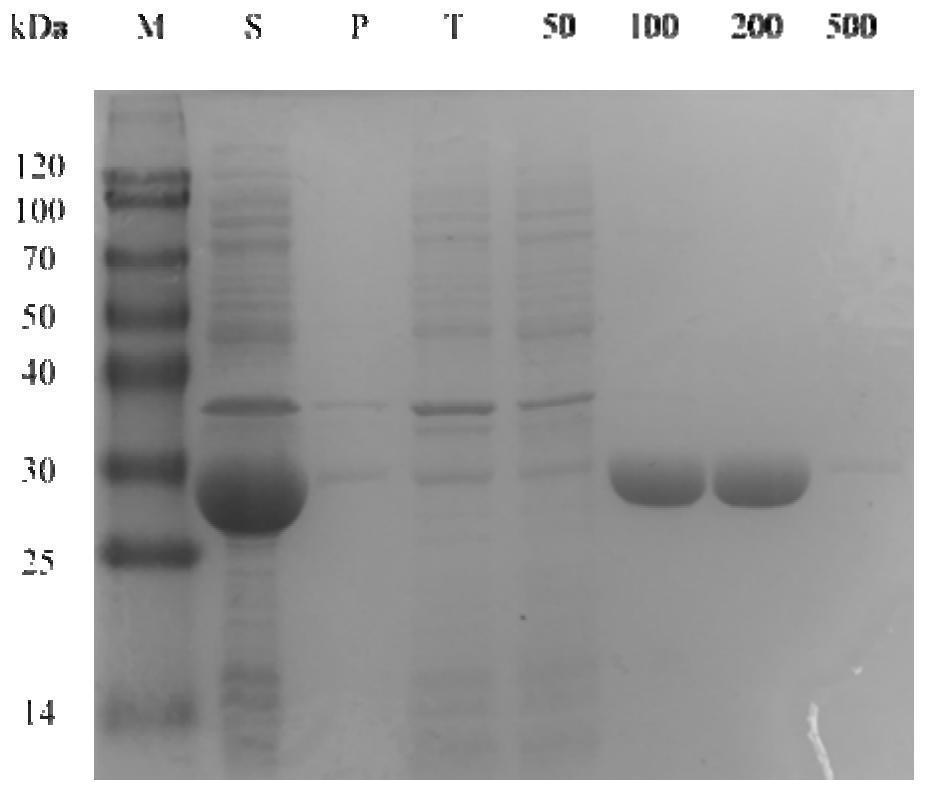
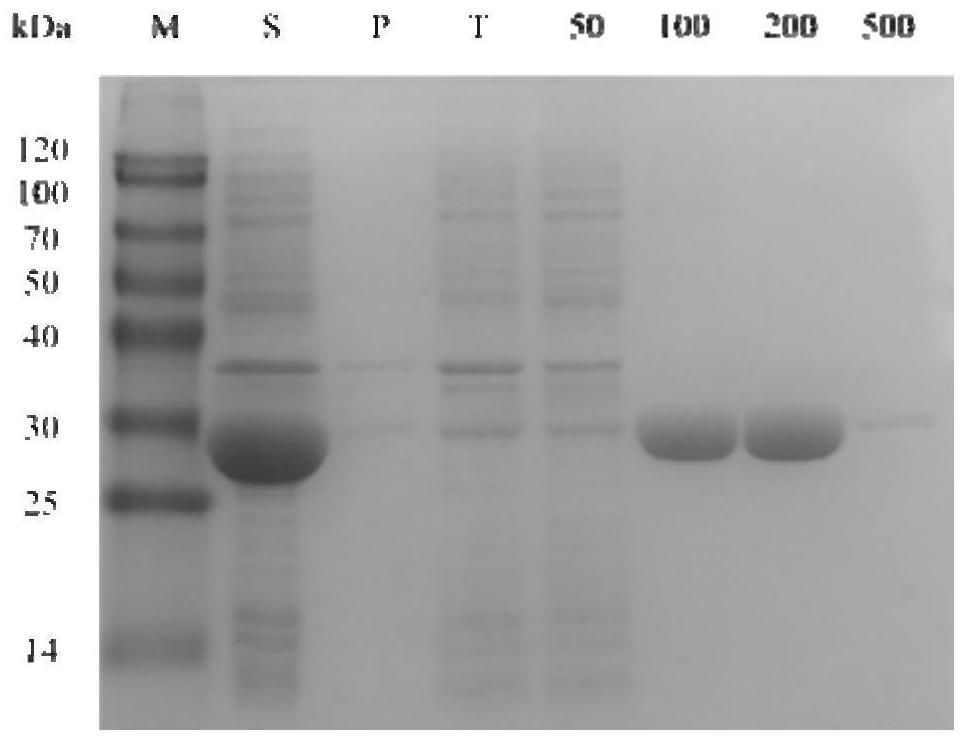
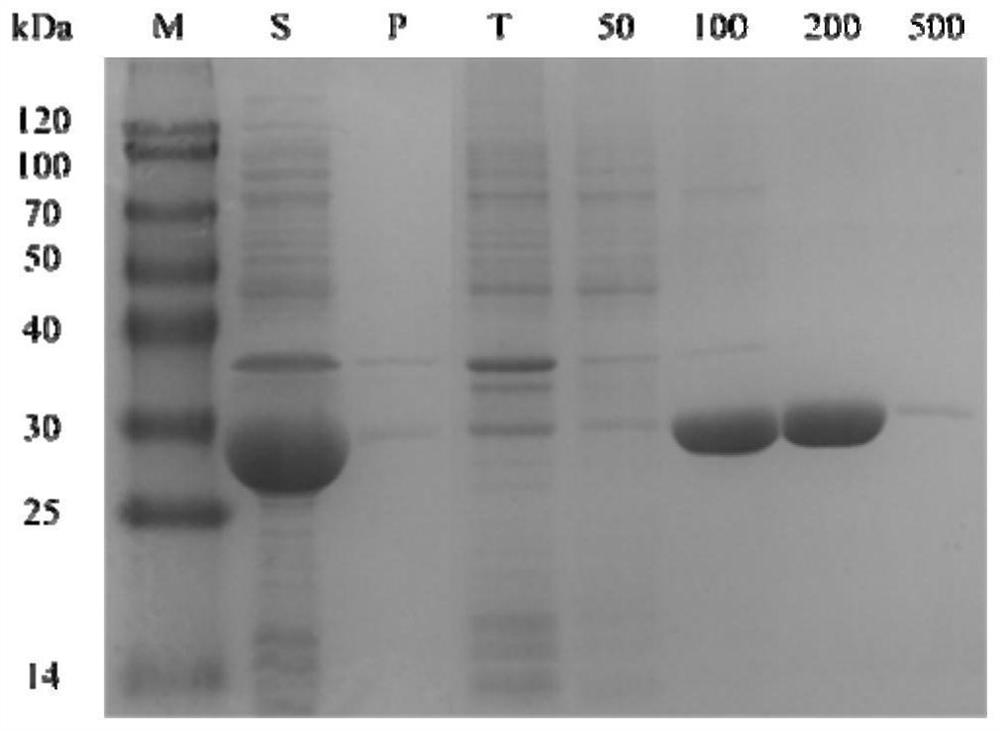
[0030] Example 1 Enzymatic activity detection and analysis stage biological reduction reaction
[0031] The corresponding lead-toteenate 2,6-dichloro-3-fluorophenylideteophenone of the target product (S) -1- (2,6-dichloro-3-fluorophenyl) ethanol is a substrate. The relative activity of the alcohol dehydrogenase CHADH90 to 2,6-dichloro-3-fluorophenylideophenone is determined by enzyme activity. The enzyme coupling circulatory system is then constructed for biological reduction. Then, the product EE value was detected to determine the stereo selectivity of the alcohol dehydrogenase.
[0032] The adjusted step of the coenzyme dependency of the alcohol dehydrogenase is: First, a crude enzyme solution of 5 μl of the alcohol dehydrogenase CHADH90, 182 μl of phosphate buffer (50 mM pH 7.0) is added to the pores of the enzyme label. 8 μlNADphPH and NADH (0.2 mm) were added to each of the adjuminations. Two min was incubated at 25 ° C. The enzyme board was placed in the enzyme label and se...
Embodiment 2
[0034] Example 2 Substrate Die and Molecular Motion Simulation
[0035] In order to obtain the spatial structure of the alcohol dehydrogenase CHADH90, the enzyme is homologous. The 3D model of the alcohol dehydrogenase CHADH90 is used with SwissModel. The 3D model of the alcohol dehydrogenase CHADH90 is from the short-chain alcohol dehydrogenase from Ralstonia Sp. DSM 6428 (PDB number: 4BMS) It is obtained for the template and the sequence similarity of both is 45%. The coenzyme NADH was laminated into the ChaDH90 model to form a CHADH90 / NADH complex. The energy minimized 2,6-dichloro-3-fluorophenylidetone was minimized to the access CHADH90 / NADH complex. According to the catalytic mechanism of the alcohol dehydrogenase, the docking results were screened, and the optimal docking posture was then selected as the initial structure as an initial structure for the initial structure. Molecular kinetic simulation.
[0036] The docking process is performed using Autodock Vina softwar...
Embodiment 3
[0038] Example 3 Construction and screening of mutants
[0039] The wild-type Chadh90 is first cultured and the plasmid of the bacteria is taken as a template for PCR. The primer is designed online with QuickChange (httore / primerdesign program.jsp). The specific method is: First, the base sequence of the alcohol dehydrogenase CHADH90 is pasted to the browser's text box; Second, click "Upload Translated", and the base sequence is automatically translated into an amino acid sequence; Third, there is a rectangular frame with a tickled in front of each amino acid after the translation, select the site you want to mutation; fourth, in the "Changeamino Acid (s) TO" location Select the target amino acid you want to suddenly Finally, click "Design Primers" system to automatically generate primers and include information such as primer length, annealing temperature.
[0040] The DNA polymerase used is a TAQ enzyme having high fidelity properties, which can achieve perfect amplification in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com