Method for preparing alpha-phenethyl alcohol by catalyzing acetophenone hydrogenation with supported amorphous alloy
An amorphous alloy and phenylethyl alcohol technology, which is applied in the field of synthesis of spices and pharmaceutical intermediates, can solve the problems of unfavorable catalyst strength, unfavorable large-scale industrial production, weak interaction between active components and carriers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (1) Roast 20-80 mesh silica gel carrier in a muffle furnace at 550°C for 5 hours, add a certain amount of saturated CuCl 2 2H 2 O solution was impregnated with an equal volume for 2 h, and then dried under vacuum at 100 °C. In an ice-water bath, 2M NaOH solution of sodium borohydride was slowly added dropwise onto the silica gel carrier impregnated with copper chloride, and stirred until no bubbles were released. The obtained catalyst was washed with distilled water, soaked with ethanol and stored in a closed container for future use. In the catalyst prepared in this embodiment, the active component copper accounts for 30% by mass percentage.
[0018] (2) In a 50mL stainless steel autoclave, sequentially add 10mL ethanol, 3g acetophenone and 0.9g supported Cu-B / SiO prepared in step (1) 2 Amorphous alloy catalyst. Seal the autoclave and pass in nitrogen gas to test for leaks, and then replace it 4 to 5 times to remove the air in the autoclave. Add hydrogen to set th...
Embodiment 2~13
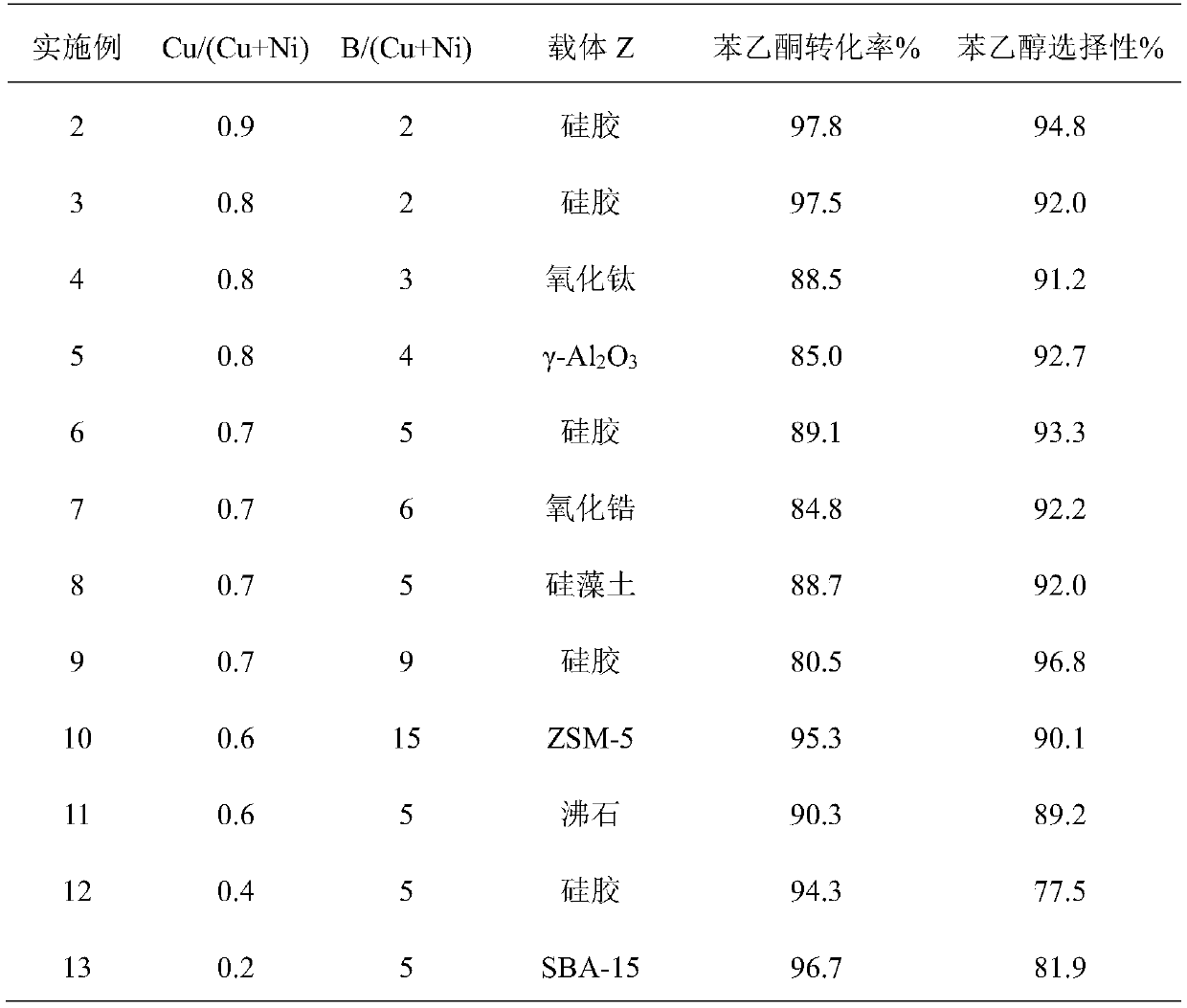
[0020] (1) Calcinate different carrier materials in a muffle furnace at 550 °C for 5 h, add a certain amount of saturated Cu(CH 3 COO) 2 ·H 2 O and Ni(CH 3 COO) 2 4H 2 The mixed solution of O was impregnated with equal volume for 8h, and then dried under vacuum at 110℃. While stirring in an ice-water bath, 2M NaOH solution of potassium borohydride was slowly added dropwise onto the support containing copper and nickel, and reacted until no bubbles were released. The obtained catalyst was washed with distilled water, soaked with ethanol, and put into a closed container for later use. In the catalyst prepared in this embodiment, the active component copper nickel accounts for 10% by mass percentage. The catalyst Cu / (Cu+Ni) atomic ratio, B / (Cu+Ni) atomic ratio and carrier selection during the preparation process are listed in Table 1.
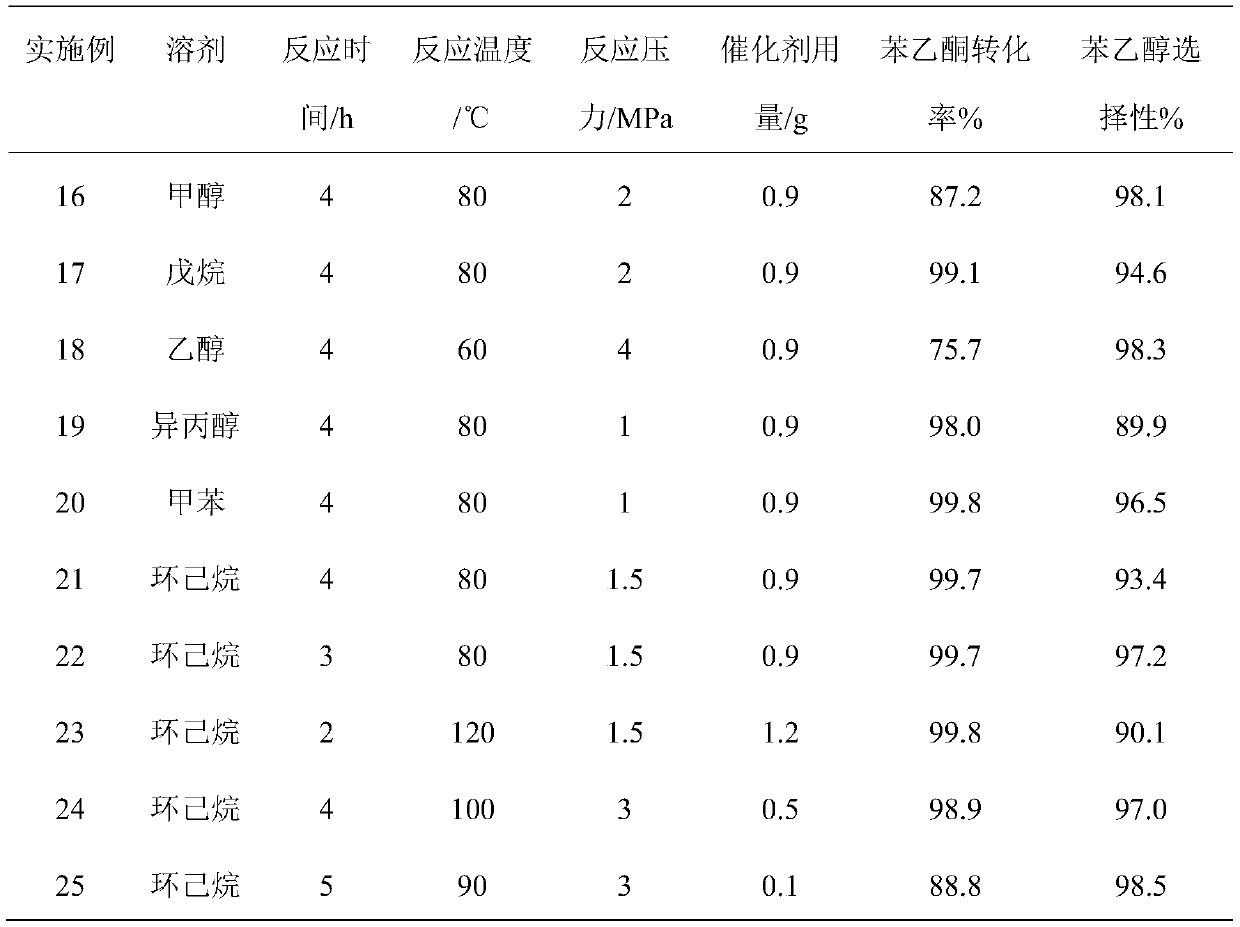
[0021] (2) In a 50 mL stainless steel autoclave, 10 mL of solvent ethanol, 3 g of acetophenone and 0.9 g of the supported Cu-Ni-B amorphou...
Embodiment 14
[0025] (1) Roast 20-60 mesh silica gel in a muffle furnace at 550°C for 5 hours, add a certain amount of saturated NiCl 2 ·6H 2 O solution was impregnated with equal volume for 24h, and then vacuum dried at 120°C. At room temperature, slowly drop 2M potassium borohydride NaOH solution onto the activated carbon impregnated with nickel chloride, and stir until no bubbles are released. The obtained catalyst was washed with distilled water, soaked with ethanol, and put into a closed container for later use. In the catalyst that present embodiment makes, by mass percentage, active component nickel accounts for 40%
[0026] (2) Prepare α-phenylethanol by hydrogenation in the same method and condition as in Example 1 step (2), the catalyst is changed to 0.9g amorphous Ni-B / SiO 2, The result of hydrogenation was 96.3% conversion of acetophenone and 60.6% selectivity of α-phenylethanol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com