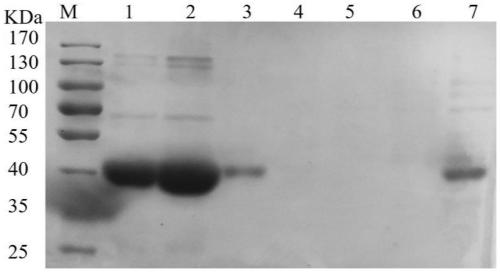
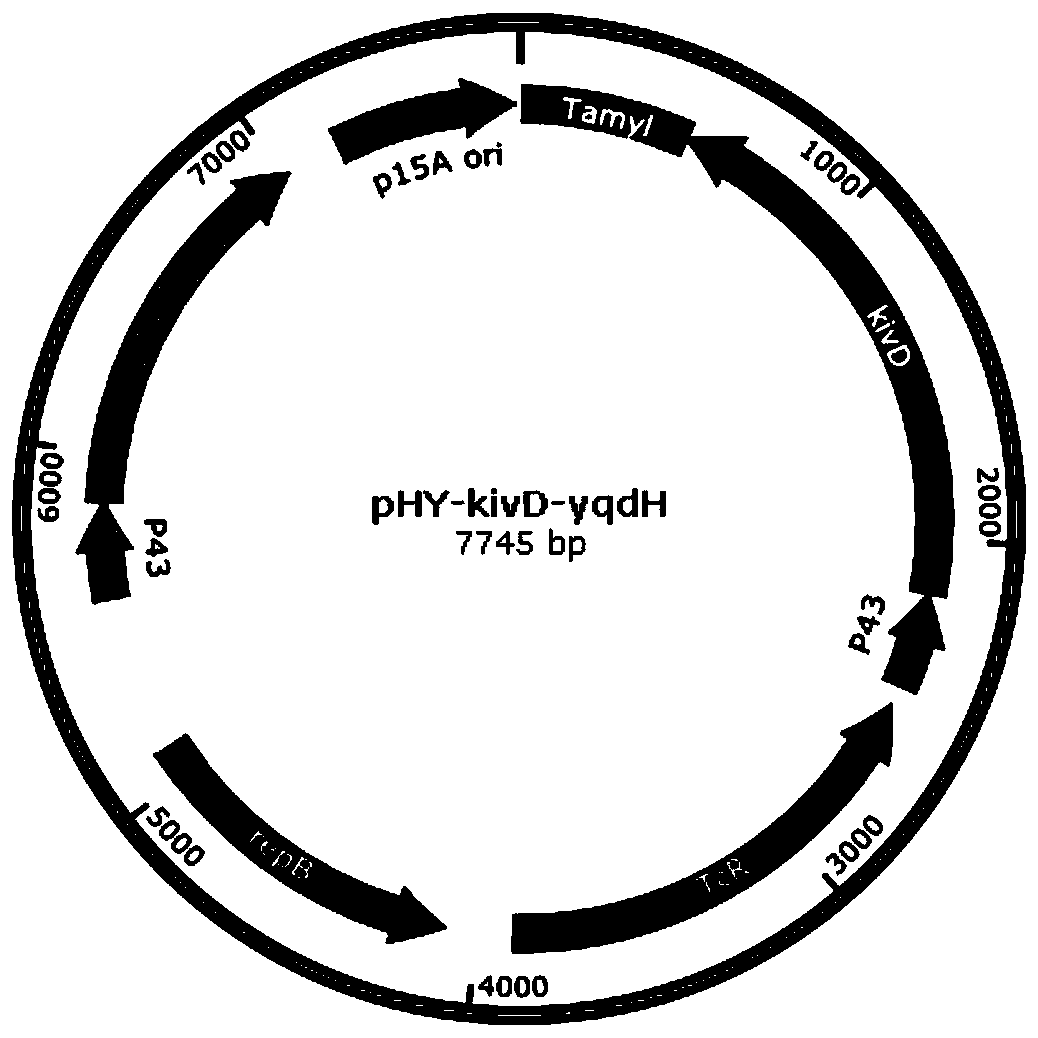
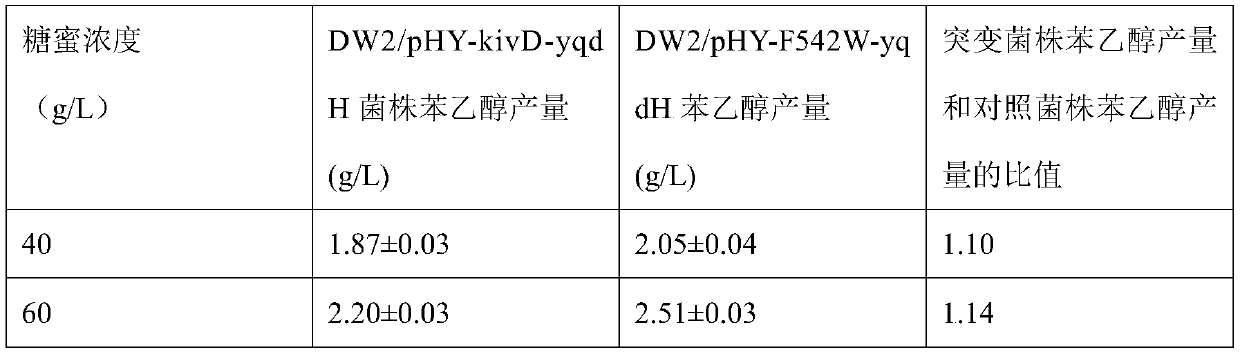
Whole-cell catalyst containing phenylpyruvic acid decarboxylase mutant and application to production of phenethyl alcohol
A technology of phenylpyruvate decarboxylase and whole-cell catalyst, which is applied in the field of bioengineering, can solve problems such as low phenylethanol tolerance, complicated operation process, and food safety issues, and achieve low catalytic efficiency, improved catalytic efficiency, The effect of increasing the conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1) Construction of recombinant plasmid pHY-kivD:
[0026] An α-ketoacid decarboxylase kivD gene was cloned from Lactococcus lactis subsp.lactis (CICC6246) by using kivD-F and kivD-R. Using the Bacillus subtilis 168 genome as a template, the P43 promoter was amplified with P43-F and P43-R primers; the amylase terminator TamyL was amplified with TamyL-F and TamyL-R primers. P43-F and TamyL-R were used as primers to perform SOE-PCR on the three fragments of P43, kivD and TamyL to obtain the fusion fragment P43-kivD-TamyL. Using the plasmid pHY300PLK as a template and pHY-T5-F and pHY-T5-R as primers, the whole plasmid was amplified by PCR to obtain a linearized pHY300PLK vector. After the above amplified products were checked by electrophoresis, the PCR products were purified and recovered using a gel recovery kit. Using the ClonExpress II one-step cloning kit, the fusion fragment was fused with the linearized vector pHY300PLK to obtain the recombinant plasmid pHY-kivD. ...
Embodiment 2
[0048] 1) Cloning of Bacillus licheniformis DW2 alcohol dehydrogenase coding gene yqdH
[0049] According to the amino acid sequence of Bacillus licheniformis alcohol dehydrogenase YqdH in the NCBI database, it is found that it has 38.65% homology with the alcohol dehydrogenase YqhD of Escherichia coli K12. YqhD is reported to have a broad substrate spectrum. In order to test the catalytic ability of Bacillus licheniformis YqdH to phenylacetaldehyde, primers yqdH-F1 and yqdH-R1 were designed according to the gene sequence of yqdH, and the genomic DNA of Bacillus licheniformis was used as a template to amplify the yqdH sequence. The alcohol dehydrogenase gene yqdH fragment was digested with BamHI and XhoI, and ligated with the expression vector pET28a(+) after the same digestion with T4DNA ligase, and the ligated product was transformed into E.coliDH5α competent cells, and the positive The clones were transferred to liquid LB medium for overnight culture, and the recombinant e...
Embodiment 3
[0058] Construction of co-expression recombinant plasmid pHY-F542W-yqdH:
[0059] Using yqdH-F2 and yqdH-R2 to amplify the yqdH gene from the Bacillus licheniformis DW2 genome, using the Bacillus subtilis 168 genome as a template and P43-F1 and P43-R as primers to amplify the P43 promoter. P43-F1 and yqdH-R2 were used as primers to perform SOE-PCR on the two fragments of P43 and yqdH to obtain the fusion fragment P43-yqdH. Using plasmid pHY-F542W or pHY-kivD as a template and amp-T5-F and amp-T5-R as primers, the whole plasmid was amplified by PCR to obtain linearized pHY-F542W and pHY-kivD. After the above amplified products were checked by electrophoresis, the PCR products were purified and recovered using a gel recovery kit. The yqdH fragments were inserted into the expression vectors pHY-F542W and pHY-kivD by the ClonExpress II one-step cloning kit, and the recombinant co-expression plasmids pHY-F542W-yqdH and pHY-kivD-yqdH were successfully constructed by colony PCR and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com